- Department of Neurosurgery, School of Medicine and Biomedical Sciences, State University of New York, USA
- Department of Neurology, School of Medicine and Biomedical Sciences, State University of New York, USA
- Department of Radiology, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, USA
- Toshiba Stroke and Vascular Research Center, University at Buffalo, State University of New York, USA
- Department of Neurosurgery, Gates Vascular Institute, Kaleida Health, New York, USA
- Jacobs Institute, Buffalo, New York, USA
- Division of Neurosurgery, Department of Surgery, The University of Arizona, Tucson, Arizona, USA
- Department of Neurosurgery, University of South Florida, Tampa, Florida, USA
Correspondence Address:
Elad I. Levy
Department of Neurosurgery, School of Medicine and Biomedical Sciences, State University of New York, USA
Department of Radiology, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, USA
Toshiba Stroke and Vascular Research Center, University at Buffalo, State University of New York, USA
Department of Neurosurgery, Gates Vascular Institute, Kaleida Health, New York, USA
DOI:10.4103/2152-7806.148847
Copyright: © 2015 Dumont TM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. A proposed grading system for endovascular treatment of cerebral arteriovenous malformations: Buffalo score. Surg Neurol Int 07-Jan-2015;6:3
How to cite this URL: Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. A proposed grading system for endovascular treatment of cerebral arteriovenous malformations: Buffalo score. Surg Neurol Int 07-Jan-2015;6:3. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=6050
Abstract
Background:The Spetzler-Martin arteriovenous malformation (AVM) grading system has proven to be useful in guiding treatment of cerebral AVMs with craniotomy. It is based on anatomical characteristics each of which makes surgical resection of an AVM more difficult, namely, deep venous drainage, eloquence of surrounding tissue, and large nidus size. A higher score correlates with more complications after treatment. Although this grading system has proven reliable over time, it does not reflect the major determinants of risk associated with endovascular treatment. The authors developed a grading system unique to endovascular treatment of cerebral AVMs.
Methods:The proposed grading system accounts for the principal AVM anatomical and physiological features that make endovascular embolization more difficult and, thus, the likelihood of complications greater. These include number of arterial pedicles, diameter of arterial pedicles, and eloquent location of AVM nidus. The proposed grading system was retrospectively applied to 50 patients undergoing endovascular AVM embolization, and its ability to predict complications was compared to the Spetzler-Martin grading system.
Results:Perioperative complications among the 50 patients included 4 major and 9 minor complications. The proposed grading system was predictive of complication risk, with an increasing rate of perioperative complications associated with an increasing AVM grade. An improved correlation of perioperative complication incidence was noted with the proposed system (P = 0.002), when compared with the Spetzler–Martin grading system (P = 0.33).
Conclusion:This grading system for the endovascular treatment of AVMs is simple, easily reproduced, and clinically valuable.
Keywords: Cerebral arteriovenous malformations, complications, endovascular glue embolization
INTRODUCTION
The arteriovenous malformation (AVM) grading system introduced by Spetzler and Martin in 1986[
Although this grading system has proven reliable over time and even in prediction of deficits after endovascular treatment,[
A grading system to estimate the risks of endovascular treatment of AVMs should take into account the anatomical difficulties unique to that therapeutic approach. We propose a grading system, the Buffalo score, that accounts for the principal anatomical and functional AVM features that we have observed to make endovascular embolization procedures more difficult and prone to complication. Such features include the number and diameter of arterial pedicles and the anatomical (functional) location of the AVM nidus.
METHODS
Description of the grading system
Important features of AVM embolization
Endovascular treatment of an AVM requires microcatheter selection of an artery feeding directly into the AVM. A liquid embolic agent is then delivered directly into the AVM directed at embolization of the abnormal vasculature. Hazards of treatment come from selective catheterization of the targeted artery or embolization of arterial supply to normal brain tissue. The proposed grading system is based on a combination of factors that make the likelihood of complication greater: Number of arterial pedicles, diameter of arterial pedicles, and eloquent location of the AVM nidus. These factors were chosen based on the operative experience of the authors and others and are discussed in detail.
Graded variables
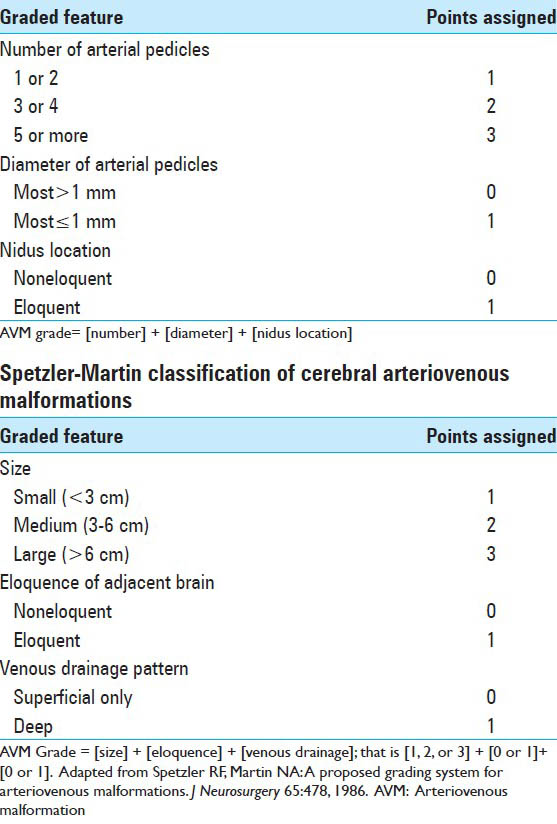
Number of arterial pedicles
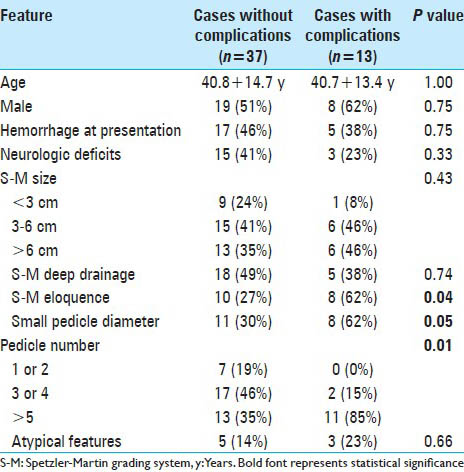
The number of arterial pedicles is estimated based on angiography. The number of arterial pedicles is determined to be 1-2, 3-4, or 5 or more and is based on an estimation of the number of arterial pedicles contributing flow into the AVM nidus. Embolization of each arterial pedicle essentially comprises a separate surgical approach as, with each embolization, the microcatheter must be removed and replaced for another arterial pedicle embolization. Whether performed during one prolonged procedure or in staged fashion, each microcatheterization and embolization carries technical risks of arterial dissection or wire perforation, as well as potential neurological damage. Thus, with an increasing number of arterial pedicles, the risk of procedural complications increases.
Arterial pedicle diameter
The diameter of arterial pedicles is estimated on the basis of angiography. Arterial pedicles are determined to be mostly large (diameter >1 mm) or small (diameter ≤1 mm). This measurement is assessed at a distal segment of the arterial pedicle, within 1 cm of the AVM nidus. A smaller vessel caliber will make catheterization-related complication more likely, as a more delicate vessel is more prone to injury during wire manipulation. In addition, reflux of glue polymer may be more likely, with less penetration into the AVM nidus owing to blood flow demand.
Eloquent location
Eloquent location of the AVM nidus is determined based on nidus location on angiography and correlated to noninvasive magnetic resonance imaging. Eloquent location of the AVM nidus is defined according to the grading system of Spetzler–Martin.[
Determination of grade
The grade of the AVM is calculated by determining the individual scores of arterial pedicle number, arterial pedicle diameter, and eloquent brain location. A numerical value is assigned to each of the categories [
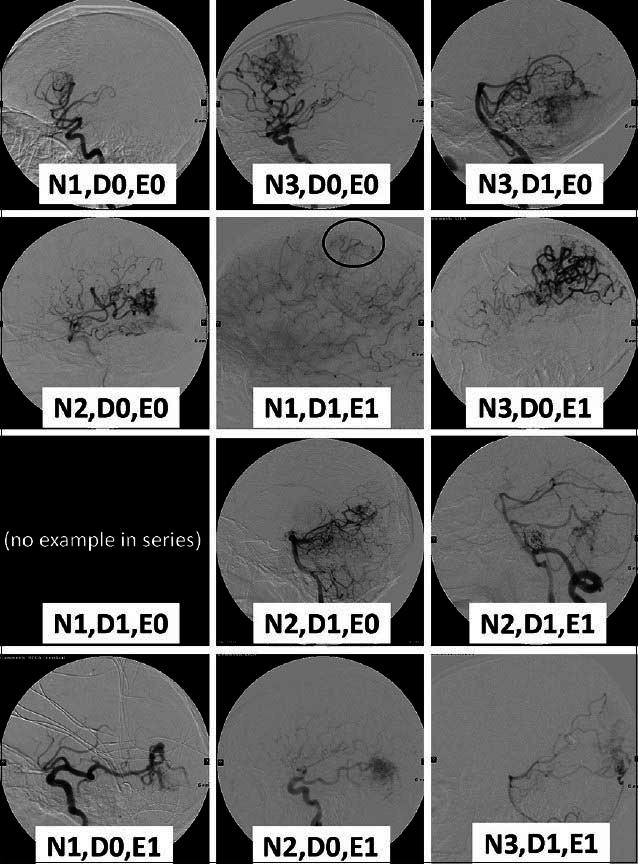
Figure 1
Lateral views of early arterial phase angiograms display examples of possible grades from the series of 50 patients retrospectively tested with the proposed grading system. No patients treated had an arteriovenous malformation (AVM) with 1 or 2 small vessels and noneloquence of adjacent brain, and thus an example of this is not shown. N: Number (of arterial pedicles); D: Diameter (of arterial pedicles); E0: Noneloquence; E1: Eloquence
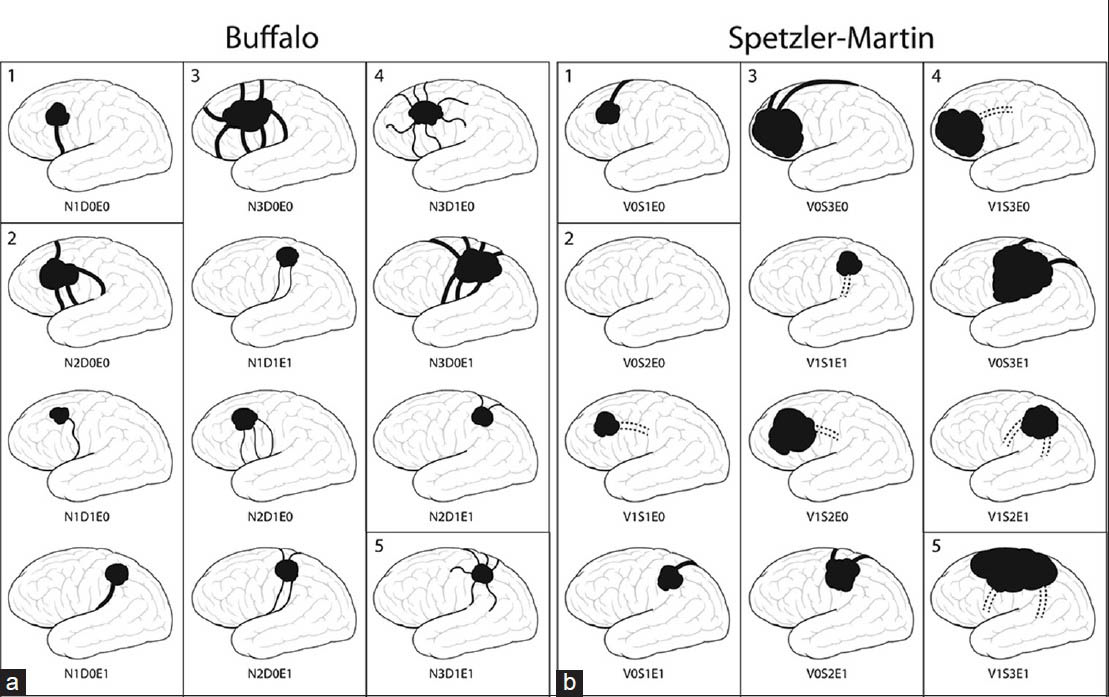
Figure 2
(a) The Buffalo system takes into account the following features: Number of arterial pedicles (N), diameter of those pedicles (D), and eloquent location (E). This schematic representation of supratentorial AVMs provides examples of different AVM types and the grade (1-5) determined by summing the points for each graded feature. In this figure, arterial pedicles and nidus are represented by black lines and shading, respectively. A higher complication incidence would be expected for patients with a higher score. See also
Application of the grading system
To test the predictive value of this grading scale, we conducted a retrospective analysis of 50 consecutive cases of AVMs in which patients were treated at our hospital between January 2006 and December 2011. Embolization was performed with the intention of cure in all cases, although in most cases this was not possible. We attempted catheterization and obliteration of all AVM pedicles until cure was achieved or when the treating physician felt that the risk of endovascular treatment was outweighed by the potential benefit, in which case adjunctive treatment with stereotactic radiosurgery or surgical resection was performed. At our hospital, endovascular embolization of one or two pedicles is generally performed at 6-week intervals for patients requiring multiple embolization procedures. Patients were generally treated under conscious sedation unless patient safety mandated intubation for the procedure. A superselective Wada test is performed to assess the eloquence of each pedicle prior to embolization, as previously described.[
Each AVM was graded according to the Spetzler–Martin scheme[
Complete obliteration of the AVM was defined as no evident contrast filling of preexistent AVM nidus and absence of early venous draining on a catheter angiogram that was performed at least 3 months after the last embolization. Cases without complete obliteration were assessed for percentage of embolization of the original nidus by two of the authors (TMD and PK). As this is a somewhat subjective measurement, no statistical assessment was made with respect to this outcome, although this data is provided as an approximate measure of completeness of obliteration in the series.
Independent variables and the grading systems were analyzed for predictive value of complications and tested for statistical significance with Fisher's Exact test or analysis of variance. Independent variables found to be significant to P < 0.20 were included in a multivariate analysis. Data were analyzed with SAS 9.2 (SAS Institute Inc., Cary, NC). The local institutional review board approved this study.
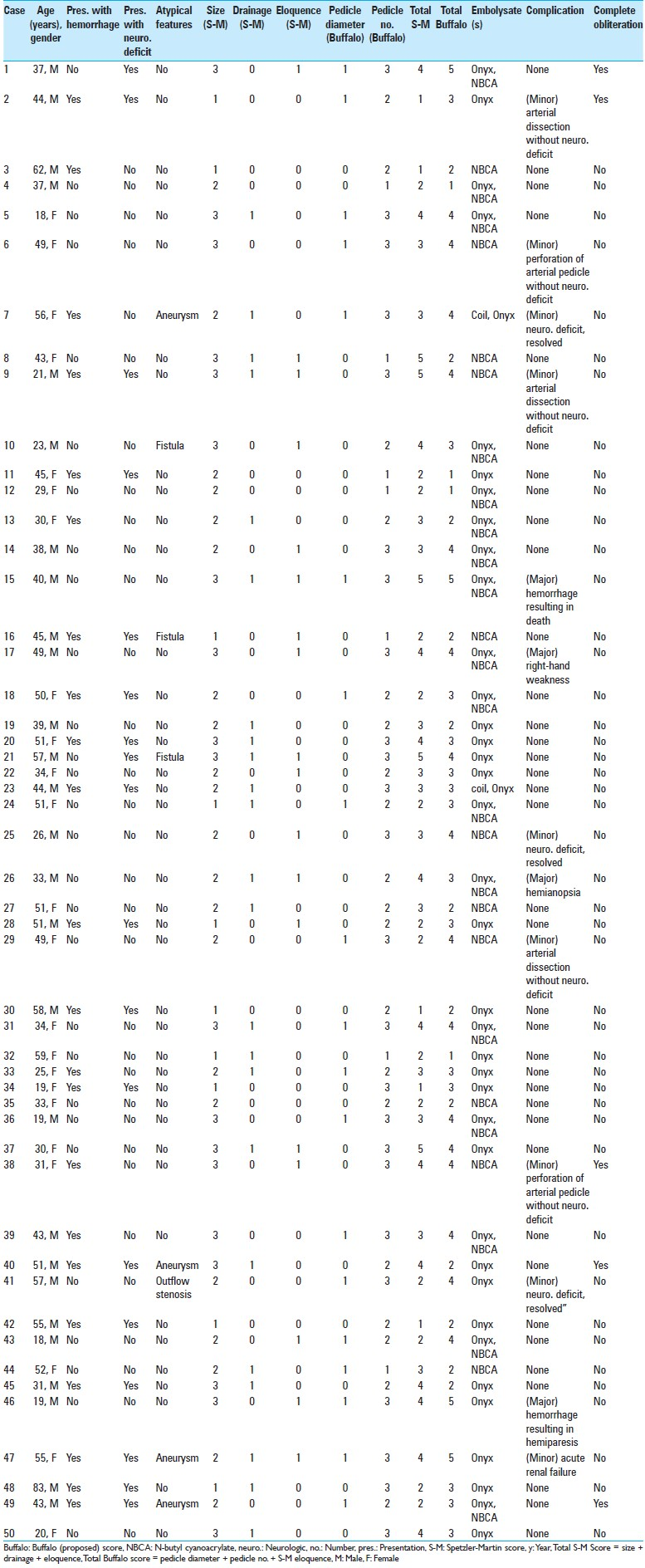
The test group consisted of 50 consecutive patients treated with endovascular AVM embolization [
RESULTS
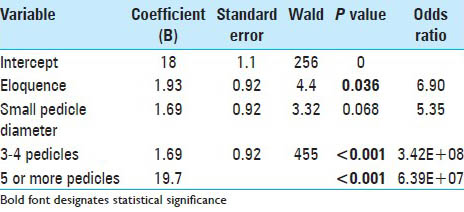
In the test group, independent variables found to be correlated with complications included number of pedicles (P = 0.01), eloquent location (P = 0.04), and small pedicle diameter (P = 0.05) [
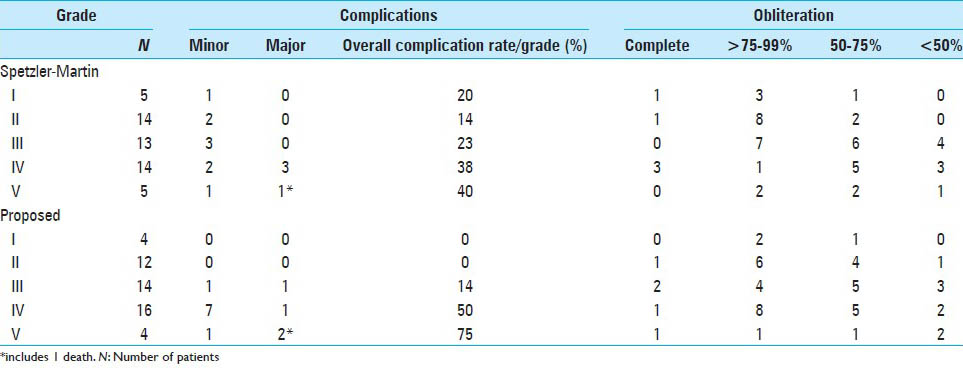
The incidence of complications was tabulated for each AVM score [
Complete obliteration was accomplished in 5 patients (10%), with >90% obliteration in 17 patients (34%) and >75% obliteration in 21 patients (42%) [
DISCUSSION
The contemporary multimodality AVM treatment paradigm has resulted in new issues in operative management. In conjunction with surgical resection of an AVM, deep venous drainage, eloquence of surrounding tissue, and nidus size are of greatest import, and thus comprise the Spetzler–Martin grading system. In endovascular treatment, we have found the diameter of arterial pedicles, number of arterial pedicles, and eloquent nidus location to be of greatest significance for complications, and thus these variables comprise the proposed grading system. The proposed grading system is simple in design, based on angiographic and magnetic resonance features, and valuable in prediction of complications.
In this series, the value of the Spetzler–Martin grading system is reduplicated for AVM treatment, as the incidence of complications was greater in grades IV and V AVMs compared with lower-grade AVMs [
The proposed grading system is based on the observed risk with endovascular treatment of AVMs. Simply stated, smaller vessels are more prone to injury with catheterization, a greater number of arterial pedicles produces more potential risk with each embolization, and eloquent location increases risk of neurological deficit. The actual size of the AVM nidus and venous drainage pattern of the AVM are of less importance during endovascular embolization but of chief importance during surgical resection. The proposed grading scheme provides a simple confluence of these concepts that will enable neurointerventionists to estimate the risk of endovascular AVM embolization.
Limitations
Limitations of the grading scheme include no prospective assessment to date and no evident correlation with complete endovascular obliteration. Of additional note, in multivariate analysis, the most relevant factor was number of arterial pedicles, whereas small pedicle diameter was not a significant factor in multivariate analysis (although it was in univariate analysis). We suspect this may be an artifact of small sample size (type II error), and we consider small vessel caliber as an important consideration when planning an embolization procedure as this feature when present typically results in more difficult microcatheterization. Furthermore, this experience is limited to a high-volume center and may not be accurate when applied universally. However, it should be noted that with the technology presently available for endovascular AVM embolization, the anticipated rate of complete obliteration is on the order of 10%.[
CONCLUSION
The proposed grading system for endovascular treatment of cerebral AVMs is simple, easily reproduced, and clinically relevant.
ACKNOWLEDGMENTS
The authors thank Paul H. Dressel BFA for preparation of the illustrations and Debra J Zimmer for editorial assistance. Dr. Dumont and Dr. Kan: Nothing to disclose. Dr. Hopkins receives grant/research support from Toshiba; serves as a consultant to Abbott, Boston Scientific, Cordis, Micrus, and Silk Road; holds financial interests in AccessClosure, Augmenix, Boston Scientific, Claret Medical, Endomation, Micrus, and Valor Medical; holds a board/trustee/officer position with Access Closure and Claret Medical; serves on Abbott Vascular's speakers’ bureau; and has received honoraria from Bard, Boston Scientific, Cleveland Clinic, Complete Conference Management, Cordis, Memorial Health Care System, and SCAI. Dr. Levy has shareholder/ownership interests in Intratech Medical Ltd., and Blockade Medical LLC. He serves as a principal investigator for the Covidien US SWIFT PRIME Trials. He receives compensation from Abbott for carotid training for physicians. Dr. Siddiqui has received research grants from the National Institutes of Health (co-investigator: NINDS 1R01NS064592-01A1, Hemodynamic induction of pathologic remodeling leading to intracranial aneurysms and co-investigator NIBIB 5RO1EB002873-07, Micro-Radiographic Image for Neurovascular Interventions), and the University at Buffalo (Research Development Award) (none of the grants are directly related to the present work); holds financial interests in Hotspur, Intratech Medical, StimSox, Valor Medical, Blockade Medical, and Lazarus Effect; serves as a consultant to Blockade Medical, Codman & Shurtleff, Inc., Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, Lazarus Effect, MicroVention, Penumbra, Inc., Stryker Neurovascular and Pulsar Vascular; belongs to the speakers’ bureaus of Codman & Shurtleff, Inc. and Genentech; serves on National Steering Committees for the following company-sponsored trials: 3D Separator (Penumbra, Inc.), FRED (Microvention), and SWIFT PRIME (Covidien); serves on advisory boards for Codman & Shurtleff and Covidien Neurovascular; and has received honoraria from Abbott Vascular and Codman & Shurtleff, Inc. for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. Dr. Siddiqui receives no consulting salary arrangements. All consulting is per project and/or per hour. Dr. Snyder serves as a consultant to, a member of the speakers’ bureau, and has received honoraria from Toshiba. He serves as a member of the speakers’ bureau for and has received honoraria from ev3/Covidien and The Stroke Group.
References
1. Feliciano CE, de Leon-Berra R, Hernandez-Gaitan MS, Rodriguez-Mercado R. A proposal for a new arteriovenous malformation grading scale for neuroendovascular procedures and literature review. P R Health Sci J. 2010. 29: 117-20
2. Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994. 34: 2-7
3. Hartmann A, Pile-Spellman J, Stapf C, Sciacca RR, Faulstich A, Mohr JP. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002. 33: 1816-20
4. Haw CS, terBrugge K, Willinsky R, Tomlinson G. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg. 2006. 104: 226-32
5. Kim LJ, Albuquerque FC, Spetzler RF, McDougall CG. Postembolization neurological deficits in cerebral arteriovenous malformations: Stratification by arteriovenous malformation grade. Neurosurgery. 2006. 59: 53-9
6. Ledezma CJ, Hoh BL, Carter BS, Pryor JC, Putman CM, Ogilvy CS. Complications of cerebral arteriovenous malformation embolization: Multivariate analysis of predictive factors. Neurosurgery. 2006. 58: 602-11
7. Pollock BE, Flickinger JC. Modification of the radiosurgery-based arteriovenous malformation grading system. Neurosurgery. 2008. 63: 239-43
8. Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002. 96: 79-85
9. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986. 65: 476-83
10. Strozyk D, Nogueira RG, Lavine SD. Endovascular treatment of intracranial arteriovenous malformation. Neurosurg Clin N Am. 2009. 20: 399-418
11. Tawk RG, Tummala RP, Memon MZ, Siddiqui AH, Hopkins LN, Levy EI. Utility of pharmacologic provocative neurological testing before embolization of occipital lobe arteriovenous malformations. World Neurosurg. 2011. 76: 276-81
12. van Beijnum J, van der Worp HB, Buis DR, Al-Shahi Salman R, Kappelle LJ, Rinkel GJ. Treatment of brain arteriovenous malformations: A systematic review and meta-analysis. JAMA. 2011. 306: 2011-9
13. Yuki I, Kim RH, Duckwiler G, Jahan R, Tateshima S, Gonzalez N. Treatment of brain arteriovenous malformations with high-flow arteriovenous fistulas: Risk and complications associated with endovascular embolization in multimodality treatment. Clinical article. J Neurosurg. 2010. 113: 715-22