- Department of Anesthesiology, Division of Neuroanesthesia and Critical Care, Trivandrum, Kerala, India
- Department of Neurosurgery, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala, India.
- Department of Nursing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala, India.
Correspondence Address:
Ajay Prasad Hrishi, Department of Anesthesiology, Division of Neuroanesthesia and Critical Care, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala, India.
DOI:10.25259/SNI_858_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ranganatha Praveen1, Manikandan Sethuraman1, Smita Vimala1, Unnikrishnan Prathapadas1, Ajay Prasad Hrishi1, Prakash Nair2, Sarath Surendran1, Arvin Ahuja1, Revikrishnan Sreekumar1, Bijith Vishnu3, Matham Gowtham2. A prospective-randomized placebo-controlled trial comparing the effects of nebulized dexmedetomidine v/s dexmedetomidine-lignocaine mixture on intraoperative hemodynamics and surgical field quality in patients undergoing endoscopic transnasal transsphenoidal pituitary tumor surgery. 15-Dec-2023;14:431
How to cite this URL: Ranganatha Praveen1, Manikandan Sethuraman1, Smita Vimala1, Unnikrishnan Prathapadas1, Ajay Prasad Hrishi1, Prakash Nair2, Sarath Surendran1, Arvin Ahuja1, Revikrishnan Sreekumar1, Bijith Vishnu3, Matham Gowtham2. A prospective-randomized placebo-controlled trial comparing the effects of nebulized dexmedetomidine v/s dexmedetomidine-lignocaine mixture on intraoperative hemodynamics and surgical field quality in patients undergoing endoscopic transnasal transsphenoidal pituitary tumor surgery. 15-Dec-2023;14:431. Available from: https://surgicalneurologyint.com/surgicalint-articles/12669/
Abstract
Background: During transnasal transsphenoidal pituitary surgery (TNTSS), the primary objective is to maintain stable hemodynamics while ensuring ideal surgical conditions. This study aimed to investigate the effect of nebulized dexmedetomidine on hemodynamic parameters and the quality of the surgical field during TNTSS.
Methods: Seventy-five patients scheduled for TNTSS were randomized into three groups of 25 each and received preoperative nebulization with 5 mL of nebulizing fluid consisting of 1.5 μg/kg of dexmedetomidine with saline in dexmedetomidine (D) group; 1.5 μg/kg of dexmedetomidine with 2% lignocaine in dexmedetomidine-lignocaine (DL) group and normal saline in the control (S) group. Heart rate (HR), mean blood pressure, Formmers score, anesthetic requirement, and emergence were evaluated for each group.
Results: Group S had significantly higher HR and mean arterial pressure than the other two groups across various time points during surgery (P P
Conclusion: The administration of nebulized dexmedetomidine, both alone and in combination with lignocaine, resulted in stable hemodynamics, favorable operative conditions, reduced anesthetic requirement, and facilitated prompt emergence during TNTSS. Nebulized dexmedetomidine proved superior to its combination with lignocaine across all evaluated parameters.
Keywords: Dexmedetomidine, Lignocaine, Nebulization, Pituitary surgery, Transnasal transsphenoidal surgery
INTRODUCTION
The anesthetic objective in transnasal transsphenoidal pituitary surgery (TNTSS) is to ensure stable hemodynamics in ideal surgical conditions, along with prompt and smooth recovery for early neurological evaluation.[
Various adjuvants, such as lignocaine and dexmedetomidine, have been shown to enhance the surgical field and reduce anesthetic requirements in TNTSS.[
MATERIALS AND METHODS
This prospective, double-blinded, and randomized controlled trial received approval from the Institutional Ethics Committee (SCT/IEC/1720/SEPTEMBER/2021) and was registered with the Clinical Trials Registry India (CTRI/2022/03/052460). The study included consenting patients aged 18–60 years presenting with pituitary tumors of any gender and classified as American Society of Anesthesiologist (ASA) physical status 1 or 2 and a Glasgow Coma Scale score (GCS) of 15 undergoing elective TNTSS. Exclusion criteria encompassed patients with GCS <15, elevated intracranial pressure, pregnancy, nursing mothers, pituitary apoplexy, and individuals with Cushing’s and Acromegaly accompanied by cardiac abnormalities. In addition, the study did not include patients with severe concurrent medical conditions such as decompensated heart failure, advanced liver disease, renal failure, medication allergies, or a history of prior nasal surgery.
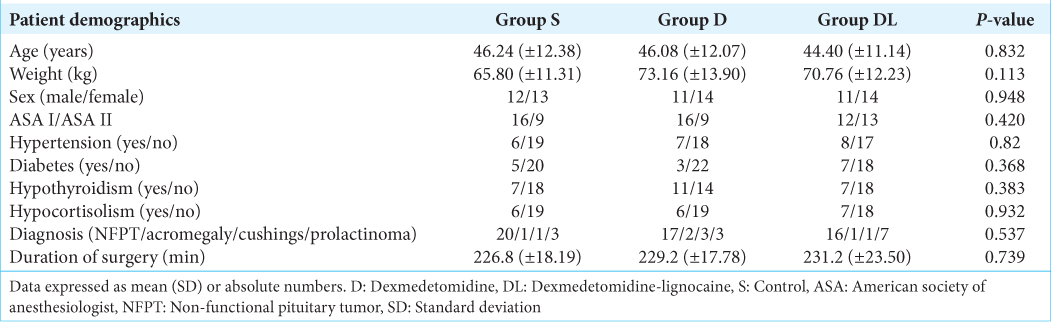
Using a computer-generated random number table, patients were allocated into three groups: the dexmedetomidine group, n = 25 (group D); the dexmedetomidine-lignocaine group, n = 25 (group DL); and the control group, n = 25 (group S) [
Anesthesia protocol
Standard ASA pre-induction monitors, such as a pulse oximeter, electrocardiogram, and non-invasive blood pressure (BP), were applied in the operating room. Radial artery cannulation was performed under local anesthesia. The patient received nebulization for 10 min, after which anesthesia was induced with injections of fentanyl (2 μg/kg) and propofol, titrated to achieve a loss of verbal response. Endotracheal intubation was facilitated using atracurium (0.5 mg/kg), and a throat pack was inserted following intubation. Anesthesia was maintained using a combination of oxygen and medical air (1:1), sevoflurane at a concentration of 0.8–1 minimum alveolar concentration (MAC), and continuous infusions of fentanyl (1–2 μg/kg/h) and atracurium (0.3 mg/kg/h) to maintain a bispectral index within the range of 40–60. All patients were ventilated to ensure normal carbon dioxide levels, and normothermia was maintained throughout the procedure.
The surgery was performed by an experienced neurosurgeon using a Mayfield 3-pin head clamp to facilitate neuronavigation. The infusions of fentanyl and atracurium were discontinued at the time of graft harvesting from the thigh for packing the sphenoid defect. In addition, ondansetron (0.1 mg/kg) and paracetamol (15 mg/kg) were administered. Sevoflurane was discontinued at the end of the surgery following the final nasal packing. The Mayfield head pins were subsequently removed, and comprehensive oral suction was performed, including removing the throat pack. Patients were ventilated with 100% oxygen, and after the reversal of neuromuscular blockade, they were extubated once it was confirmed that they exhibited sufficient respiratory efforts and appropriate response to simple commands. Subsequently, patients were monitored in the intensive care unit for the following 24 hours.
Study protocol
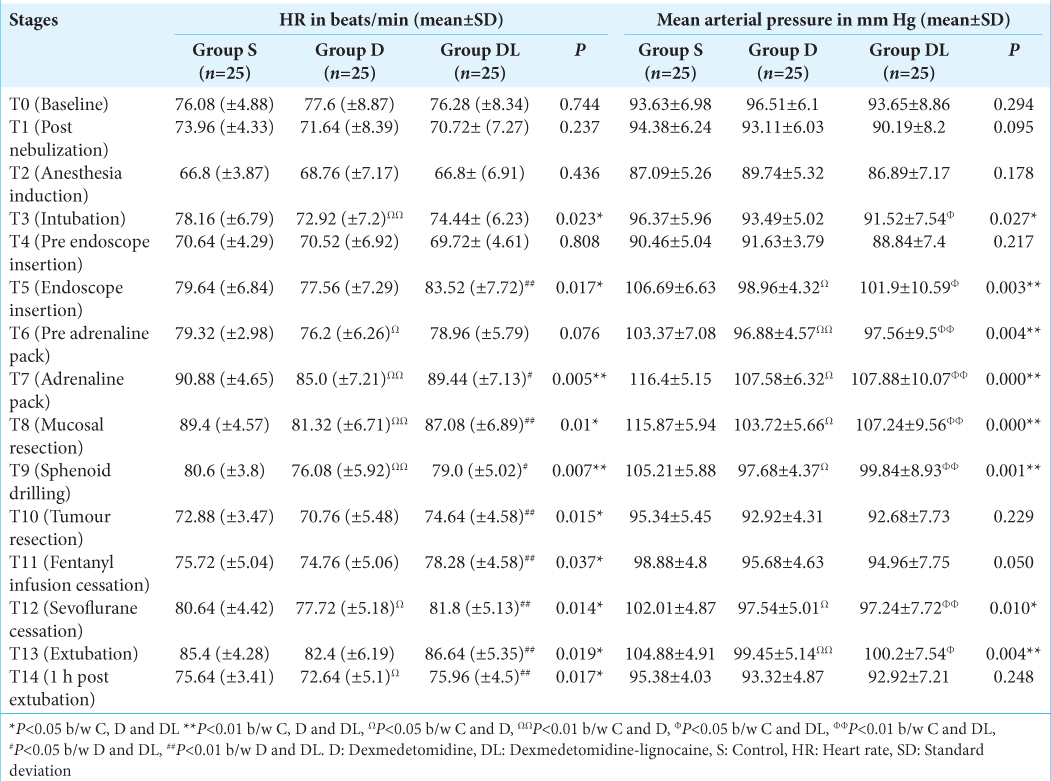
Hemodynamic parameters, including heart rate (HR), systolic BP, diastolic BP, and mean BP (MBP), were monitored at various time points during anesthesia and surgery, denoted as follows: T0 (Baseline), T1 (post-nebulization), T2 (post-anesthesia induction), T3 (post-intubation), T4 (pre-nasal endoscope insertion), T5 (at endoscope insertion), T6 (before adrenaline pack placement), T7 (during adrenaline packing), T8 (during mucosal resection), T9 (during sphenoid drilling), T10 (during tumor resection), T11 (after discontinuing fentanyl infusion), T12 (after stopping inhalational agent), T13 (after extubation), and T14 (30 min post-extubation).
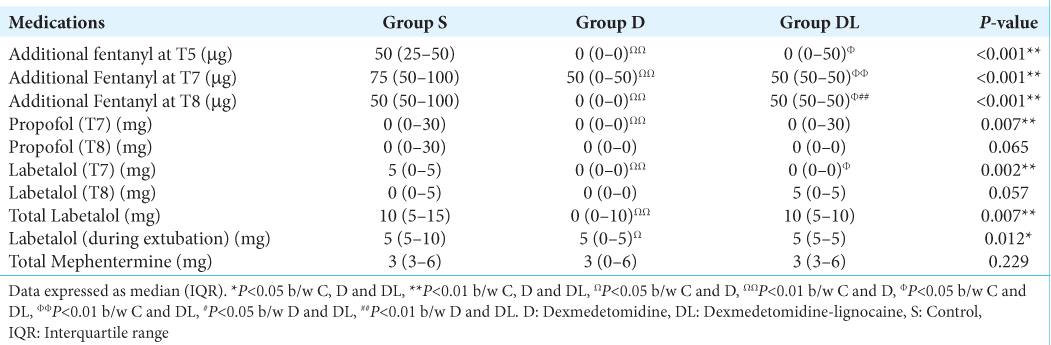
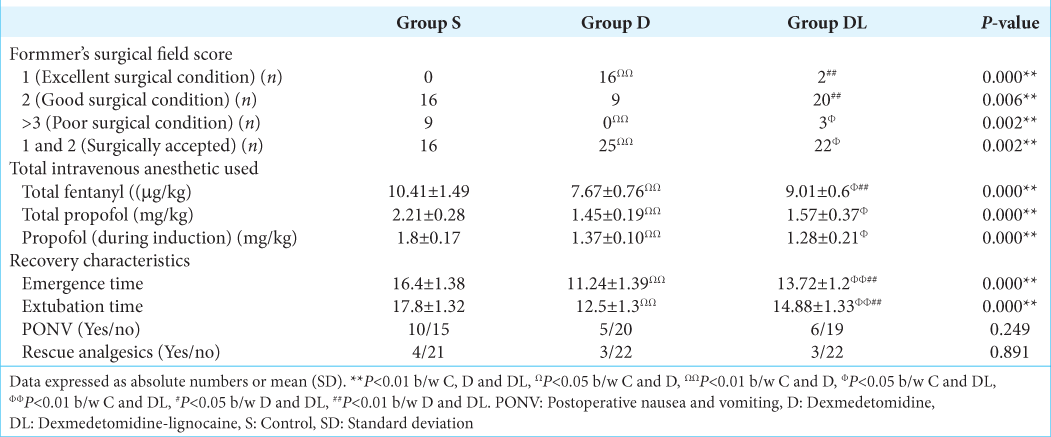
In the event of MBP or HR > 20% of the baseline, additional intravenous bolus doses of fentanyl (50 µg/bolus) were administered. If elevated BP or HR values persisted, additional intravenous boluses of propofol (30 mg/bolus) and labetalol (5 mg/bolus) were given, and the sevoflurane MAC was increased up to 1. These boluses were repeated to maintain optimal hemodynamics (HR and BP within 20% of baseline). Intravenous mephentermine boluses (3 mg/bolus) were administered when BP < 20% of baseline, and atropine (0.6 mg) was prepared for administration if HR dropped below 40 beats/min. Total doses of fentanyl and propofol (during intubation and head clamping), fentanyl infusion, and additional amounts of propofol and fentanyl at different time points were documented. The use of labetalol, mephentermine, and sevoflurane MAC at various time points was also recorded. Emergence and extubation times were noted, as well as postoperative nausea and vomiting (PONV), pain, and the need for supplementary analgesia during the 24-hour postoperative period. The surgical field quality during the procedure was evaluated by surgeons using Formmer’s scores, with a score of one considered excellent, one and two as acceptable, and three and above as indicative of unacceptable surgical conditions.
Statistical analysis
We conducted the statistical analysis using SPSS Inc., Chicago, IL, version 26.0. Continuous variables were presented as mean ± standard deviation or median (interquartile range) for skewed data, while categorical data were described using frequency, ratio, and percentage (%). We employed one-way analysis of variance (ANOVA) and the Kruskal–Wallis test to compare continuous and categorical parameters between groups. ANOVA for repeated measures was used to compare continuous variables within the groups. P < 0.05 was considered statistically significant for intergroup comparisons.
The sample size was determined based on the available literature, specifically regarding Formmer’s score from a prior study. In this study, the proportion of outcomes with Formmer’s scores >3 in the control group was 53%, whereas in the treatment group, it was 16%.[
RESULTS
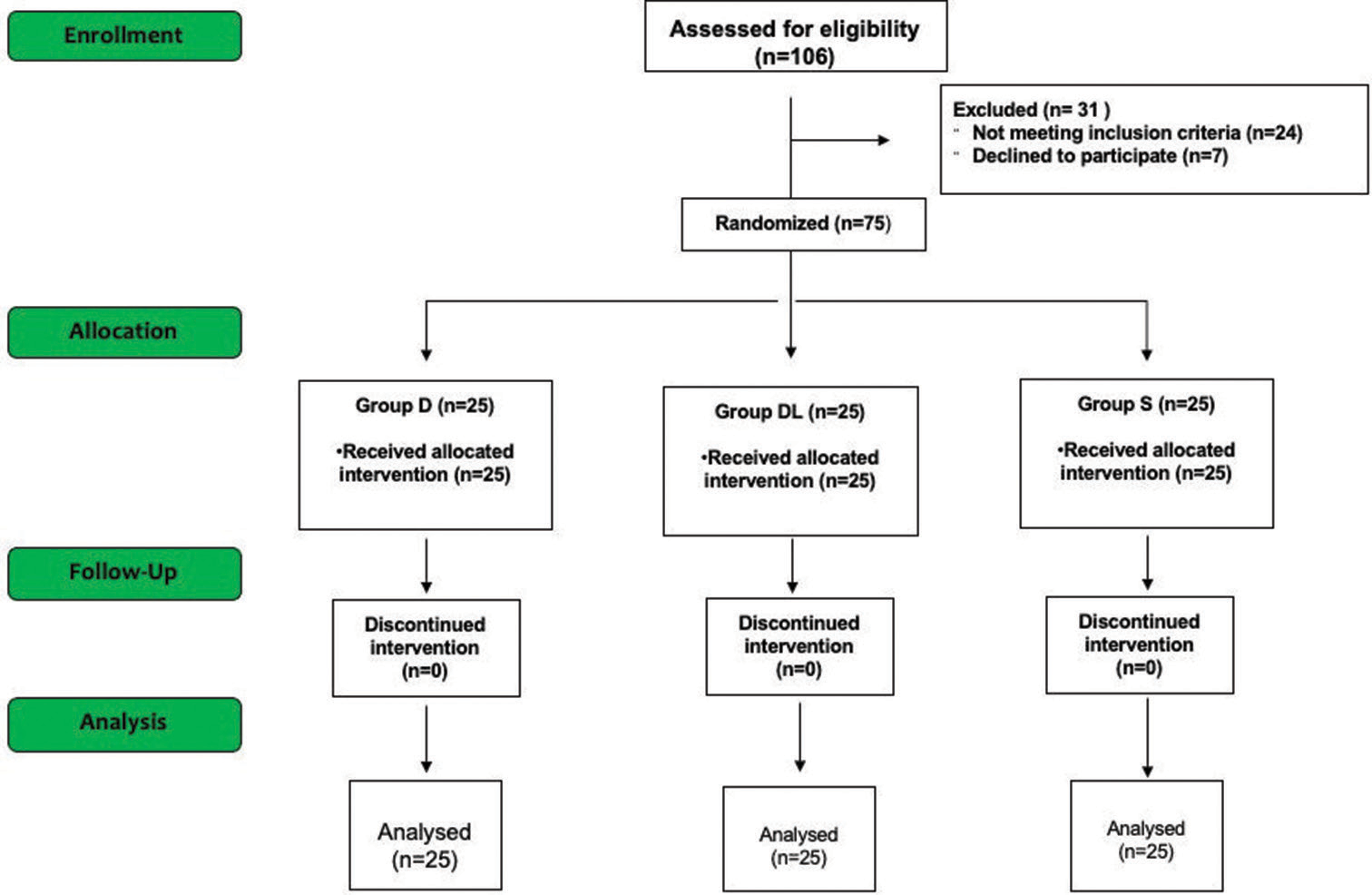
Overall, 106 patients were screened for eligibility; 24 did not meet the inclusion criteria, and seven declined participation. Other 75 patients who participated in the study were randomized into the S, D, and DL groups, comprising 25 patients in each group. All these patients completed the study [
The S group, compared to the D and DL groups, required additional doses of fentanyl to attenuate the stress responses during endoscope insertion (T5), adrenaline packing (T7), and mucosal resection (T8) [
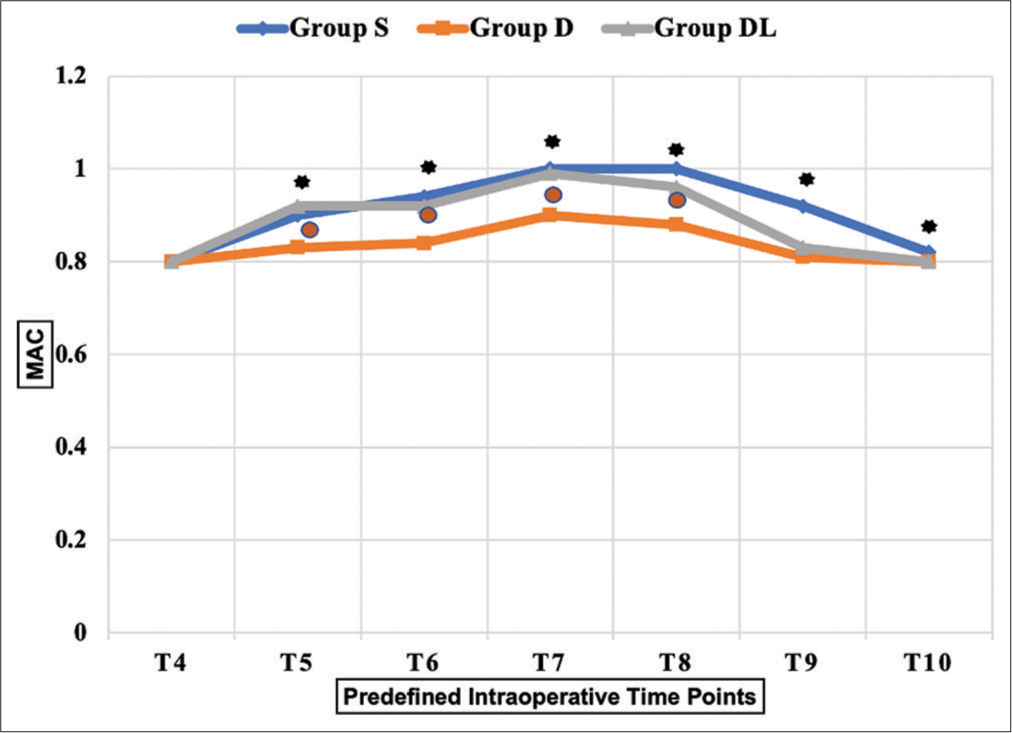
The S group required a significantly higher MAC to maintain optimal hemodynamics than the D group from endoscopic insertion until tumor resection (T5 to T10) (P < 0.01). Moreover, a higher MAC of sevoflurane was needed in the S group compared to the DL group during the T8 and T9 time frames. The DL group required a significantly higher MAC from T5 to T8 than the D group [
In the D group, 16 patients (64%) achieved a grade one Formmer score, as compared to two patients (8%) in the DL group and none in the S group, which was statistically significant (P = 0.000). A combination of grade 1 and grade 2 Formmer scores considered an acceptable surgical field was observed in all patients (100%) in the D group versus 22 patients (88%) in the DL group versus 16 patients (64%) in the S group. Formmer scores of >3, indicating a compromised surgical field, were found to be significantly higher in groups S and DL, with nine patients (32%) in the S group, three patients (12%) in the DL group, and none in the D group [
DISCUSSION
Our study marks the first attempt to assess the impact of nebulized dexmedetomidine, either alone or in combination with lignocaine, on the surgical field, hemodynamic stability, and anesthetic requirements in TNTSS. We discovered that pre-anesthesia nebulization of dexmedetomidine or its combination with lignocaine in TNTSS patients resulted in several favorable outcomes compared to saline nebulization. These outcomes included optimal intraoperative hemodynamic parameters, improved surgical conditions, reduced anesthetic requirements, and facilitated early emergence and extubation.
One of the distinctive challenges in endoscopic TNTSS, compared to endoscopic nasal surgeries, is the need to maintain sufficient cerebral blood flow (CBF) and ensure early emergence for neurological assessment. Induced hypotension, often used to minimize bleeding in endoscopic nasal surgery, can jeopardize CBF in TNTSS.[
Dexmedetomidine has been safely used as an adjuvant in central neuraxial and peripheral nerve blocks, with substantial bioavailability when absorbed through the nasal and buccal mucosa.[
The patients in the control group experienced a significant increase in MAP from the baseline during adrenaline packing and mucosal resection, necessitating higher sevoflurane MAC, additional doses of fentanyl, propofol, and labetalol to maintain stable hemodynamics. However, patients who received nebulized dexmedetomidine alone or in combination with lignocaine maintained stable hemodynamics throughout the surgery. In addition, the total requirement of fentanyl, propofol, and sevoflurane was significantly lower in these groups as compared to the control group. The sympatholytic properties of dexmedetomidine could have blunted the response to noxious stimuli during various surgical stages, resulting in a significantly reduced requirement for anesthetics.[
In our study, patients in the dexmedetomidine groups had optimal surgical fields, with a substantial percentage of patients having excellent Formmers grades. In contrast, many patients in the control group experienced poor surgical conditions. Dexmedetomidine’s ability to attenuate stress-induced hypertension also aided in minimizing surgical field bleeding, resulting in better operating conditions.[
Intravenous dexmedetomidine during TNTSS has been associated with a lower incidence of PONV and reduced need for postoperative rescue analgesics.[
Our findings indicate that the D group outperformed the DL group in terms of stable hemodynamic profile, providing an excellent surgical field with reduced anesthetic requirement and emergence time. This difference may be attributed to the altered behavior of nebulized particles of the two compatible drug preparations, which may differ from the properties of individually nebulized drug preparation particles.[
Limitations
While most of the cases were operated on by the same surgeon, a small number were operated on by other surgeons with more than five years of experience in TNTSS. We included patients with a good ASA grade and normal cardiac function, and further studies including higher ASA grade patients with borderline cardiac function will help confirm the advantages described in our research over intravenous dexmedetomidine in TNTSS. In our study, lignocaine and dexmedetomidine were mixed and nebulized to save time. Nebulizing lignocaine and dexmedetomidine separately might yield different results when comparing the dexmedetomidine alone and combination groups.
CONCLUSION
Administering nebulized dexmedetomidine at a dose of 1.5 µg/kg, both alone and in combination with lignocaine, ensured stable hemodynamics and favorable surgical conditions during TNTSS surgery. In addition, it reduced the need for anesthetics and promoted a prompt and seamless emergence from anesthesia in this group of patients. Notably, nebulized dexmedetomidine outperformed its combination with nebulized lignocaine in achieving excellent surgical conditions, reducing the need for anesthetics, and facilitating early post-anesthesia recovery.
Ethical approval
The author(s) declare that they have taken the ethical approval from IEC - SCT/IEC/1720/SEPTEMBER/ 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdallah MY, Khafagy YW, AbdAllah MY. Efficacy of dexmedetomidine versus propofol in patients undergoing endoscopic transnasal transsphenoidal pituitary tumor resection. Anesth Essays Res. 2021. 15: 368-74
2. Abdel-Ghaffar HS, Kamal SM, El Sherif FA, Mohamed SA. Comparison of nebulised dexmedetomidine, ketamine, or midazolam for premedication in preschool children undergoing bone marrow biopsy. Br J Anaesth. 2018. 121: 445-52
3. Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012. 62: 118-33
4. Ali Z, Prabhakar H, Bithal PK, Dash HH. Bispectral index-guided administration of anesthesia for transsphenoidal resection of pituitary tumors: A comparison of 3 anesthetic techniques. J Neurosurg Anesthesiol. 2009. 21: 10-5
5. Altermatt FR, Bugedo DA, Delfino AE, Solari S, Guerra I, Muñoz HR. Evaluation of the effect of intravenous lidocaine on propofol requirements during total intravenous anaesthesia as measured by bispectral index. Br J Anaesth. 2012. 108: 979-83
6. Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003. 56: 691-3
7. Bala R, Chaturvedi A, Pandia MP, Bithal PK. Intraoperative dexmedetomidine maintains hemodynamic stability and hastens postoperative recovery in patients undergoing transsphenoidal pituitary surgery. J Neurosci Rural Pract. 2019. 10: 599-605
8. Bharti N, Praveen R, Bala I. A dose-response study of caudal dexmedetomidine with ropivacaine in pediatric day care patients undergoing lower abdominal and perineal surgeries: A randomized controlled trial. Paediatr Anaesth. 2014. 24: 1158-63
9. Bharti N, Sardana DK, Bala I. The analgesic efficacy of dexmedetomidine as an adjunct to local anesthetics in supraclavicular brachial plexus block: A randomized controlled trial. Anesth Analg. 2015. 121: 1655-60
10. Dehghan MH, Gaikwad VM, Dandge B. Nasal absorption of drugs-barriers and solutions. Res J Pharm Tech. 2009. 2: 634-41
11. Geetha K, Padhy S, Karishma K. Comparison of single-shot nebuliser protocol between dexmedetomidine and ketamine in children undergoing magnetic resonance imaging. J Perioper Pract. 2022. 32: 346-53
12. Gopalakrishna KN, Dash PK, Chatterjee N, Easwer HV, Ganesamoorthi A. Dexmedetomidine as an anesthetic adjuvant in patients undergoing transsphenoidal resection of pituitary tumor. J Neurosurg Anesthesiol. 2015. 27: 209-15
13. Groeben H, Mitzner W, Brown RH. Effects of the alpha2-adrenoceptor agonist dexmedetomidine on bronchoconstriction in dogs. Anesthesiology. 2004. 100: 359-63
14. Gu W, Xu M, Lu H, Huang Q, Wu J. Nebulized dexmedetomidine-lidocaine inhalation as a premedication for flexible bronchoscopy: A randomized trial. J Thorac Dis. 2019. 11: 4663-70
15. Hrishi PA, Ruby K, Nair P. A novel use of a novel drug: Preoperative nasal preparation with dexmedetomidine fortransnasal transsphenoidal neurosurgery approach in skull base neurosurgery. Indian J Neurosurg. 2017. 6: 170-5
16. Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011. 67: 825-31
17. Jain D, Bhagat H, Jain D. Effect of intravenous lignocaine infusion on the quality of emergence in patients undergoing transsphenoidal resection of pituitary tumors-a prospective, randomized controlled trial. Surg Neurol Int. 2020. 11: 154
18. Jan S, Ali Z, Nisar Y, Naqash IA, Zahoor SA, Langoo SA. A comparison of dexmedetomidine and clonidine in attenuating the hemodynamic responses at various surgical stages in patients undergoing elective transnasal transsphenoidal resection of pituitary tumors. Anesth Essays Res. 2017. 11: 1079-83
19. Kamin W, Schwabe A, Krämer I. Inhalation solutions: Which one are allowed to be mixed? Physico-chemical compatibility of drug solutions in nebulizers. J Cyst Fibros. 2006. 5: 205-13
20. Kang R, Jeong JS, Ko JS, Lee SY, Lee JH, Choi SJ. Intraoperative dexmedetomidine attenuates norepinephrine levels in patients undergoing transsphenoidal surgery: A randomized, placebo-controlled trial. BMC Anesthesiol. 2020. 20: 100
21. Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999. 54: 146-65
22. Konay AS, Williams A, Chacko AG, Singh G. A prospective randomized trial comparing topical intranasal lidocaine and levobupivacaine in patients undergoing endoscopic binostril transnasal transsphenoidal resection of pituitary tumors. J Neurosurg Anesthesiol. 2021. 33: 51-7
23. Kumar A, Kumari P, Sinha C, Kumar A, Kumar R, Kumar A. Dexmedetomidine nebulization as adjuvant to lignocaine during awake flexible fiberoptic intubation. Saudi J Anaesth. 2019. 13: 152-3
24. Kumar NR, Jonnavithula N, Padhy S, Sanapala V, Naik VV. Evaluation of nebulised dexmedetomidine in blunting haemodynamic response to intubation: A prospective randomised study. Indian J Anaesth. 2020. 64: 874-9
25. Mathew PJ, Jain A, Wig J, Mukherjee KK, Gupta A. Randomized controlled trial of intravenous dexmedetomidine for control of hemodynamics, surgical bleeding, and recovery profile during transsphenoidal pituitary surgery. J Neuroanaesth Crit Care. 2020. 7: 10
26. Mishra P, Kaushik M, Dehadaray A, Qadri H, Raichurkar A, Seth T. Preparation of nose for nasal endoscopy: Cotton pledget packing versus topical spray. A prospective randomized blinded study. Eur Arch Otorhinolaryngol. 2013. 270: 117-21
27. Misra S, Behera BK, Mitra JK, Sahoo AK, Jena SS, Srinivasan A. Effect of preoperative dexmedetomidine nebulization on the hemodynamic response to laryngoscopy and intubation: A randomized control trial. Korean J Anesthesiol. 2021. 74: 150-7
28. Moeen SM, Mandour AM, Soliman OM, Osman MM. Effect of lidocaine infusion on intraoperative bleeding during functional endoscopic sinus surgery: A randomized controlled trial. Minerva Anestesiol. 2022. 88: 901-9
29. Niyogi S, Biswas A, Chakraborty I, Chakraborty S, Acharjee A. Attenuation of haemodynamic responses to laryngoscopy and endotracheal intubation with dexmedetomidine: A comparison between intravenous and intranasal route. Indian J Anaesth. 2019. 63: 915-23
30. O’Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997. 52: S31-44
31. Salimi A, Sharifi G, Bahrani H, Mohajerani SA, Jafari A, Safari F. Dexmedetomidine could enhance surgical satisfaction in trans-sphenoidal resection of pituitary adenoma. J Neurosurg Sci. 2017. 61: 46-52
32. Sawka AM, Aniszewski JP, Young WF, Nippoldt TB, Yanez P, Ebersold MJ. Tension pneumocranium, a rare complication of transsphenoidal pituitary surgery: Mayo clinic experience 1976-1998. J Clin Endocrinol Metab. 1999. 84: 4731-4
33. Sharma BS, Sawarkar DP, Suri A. Endoscopic pituitary surgery: Techniques, tips and tricks, nuances, and complication avoidance. Neurol India. 2016. 64: 724-36
34. Sharma GK, Verma SP. Is nebulized lidocaine adequate topical anesthesia for diagnostic transnasal tracheoscopy?. Ann Otol Rhinol Laryngol. 2015. 124: 545-9
35. Soliman R, Fouad E. The effects of dexmedetomidine and magnesium sulphate in adult patients undergoing endoscopic transnasal transsphenoidal resection of pituitary adenoma: A double-blind randomised study. Indian J Anaesth. 2017. 61: 410-7
36. Sonbol AM, Ahmed SG. Comparative evaluation of dexmedetomidine versus magnesium sulphate on the adequacy of hypotensive anesthesia and postoperative recovery for patients undergoing endoscopic transnasal transsphenoidal pituitary tumor resection. Egypt J Hosp Med. 2022. 86: 59-65
37. Sriramka B, Warsi ZH, Sahoo J. Effects of adding dexmedetomidine to nebulized lidocaine on control of hemodynamic responses to laryngoscopy and intubation: A randomized clinical trial. J Anaesthesiol Clin Pharmacol. 2023. 39: 11-7
38. Tang C, Huang X, Kang F, Chai X, Wang S, Yin G. Intranasal dexmedetomidine on stress hormones, inflammatory markers, and postoperative analgesia after functional endoscopic sinus surgery. Mediators Inflamm. 2015. 2015: 939431
39. Zanaty OM, El Metainy SA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination as premedication for outpatient pediatric dental surgery. Anesth Analg. 2015. 121: 167-71