- Department of Medicine, Federal University of Sergipe, Aracaju, Brazil
- Department of Medicine, Universidade Tiradentes, Aracaju, Brazil
- Department of Neurosurgery, Hospital de Cirurgia, Aracaju, Brazil
Correspondence Address:
Arthur Maynart Pereira Oliveira, Department of Neurosurgery, Hospital de Cirurgia, Aracaju, Brazil.
DOI:10.25259/SNI_134_2025
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vittor Sérgio Santos de Quintela1, Sofia Morais Silva Almeida1, Arthur Campos do Nascimento1, Nelson Almeida D’Ávila Melo2, Arthur Maynart Pereira Oliveira3. A rare case of giant infrasellar craniopharyngioma with extensive invasion of the pterygopalatine fossa: A case report and literature review. 02-May-2025;16:161
How to cite this URL: Vittor Sérgio Santos de Quintela1, Sofia Morais Silva Almeida1, Arthur Campos do Nascimento1, Nelson Almeida D’Ávila Melo2, Arthur Maynart Pereira Oliveira3. A rare case of giant infrasellar craniopharyngioma with extensive invasion of the pterygopalatine fossa: A case report and literature review. 02-May-2025;16:161. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13535
Abstract
BackgroundCraniopharyngiomas are benign epithelial tumors that arise along the craniopharyngeal duct, commonly located in the sellar or suprasellar region. Infrasellar extension is a rare variant and may involve the nasopharynx, sphenoid sinus, clivus, and pterygopalatine fossa.
Case DescriptionA 66-year-old male patient is presented to the otorhinolaryngology service due to a complaint of left ear obstruction for the past 4 months. After no response to clinical treatment, investigation with computed tomography and magnetic resonance imaging showed a heterogeneous lesion with areas of calcification and bone destruction located in the sphenoid sinus region, which projected inferiorly and laterally invading the clivus in its entirety, the petrous apex, middle fossa, pterygopalatine, and infratemporal fossae with no involvement of the sellar/suprasellar region. The patient was referred to a multidisciplinary skull base surgery group that performed an extended transpterygoid endoscopic endonasal approach with gross total resection. The anatomopathological study was consistent with adamantinomatous craniopharyngioma.
ConclusionWe present a rare case of a giant infrasellar craniopharyngioma with extensive invasion of the skull base without involvement of the sella or the pituitary gland.
Keywords: Case report, Craniopharyngioma, Literature review, Skull base neoplasms, Sphenoid sinus
INTRODUCTION
Craniopharyngiomas are benign epithelial tumors that arise along the craniopharyngeal duct.[
Craniopharyngiomas usually grow along the vertical axis from the sella to the third ventricle.[
Isolated infrasellar craniopharyngiomas without sellar and pituitary involvement are exceedingly rare. In this case report and literature review, we will scrutinize an infrasellar craniopharyngioma, an uncommon incidence, discussing its clinical and diagnostic characteristics, as well as the treatment approaches.
CASE REPORT
A 66-year-old male patient with a medical history of diabetes mellitus using oral hypoglycemic agents, without other comorbidities or prior surgeries, sought the otorhinolaryngology (ORL) service due to the sensation of aural fullness on the left for approximately 4 months. In the initial investigation with the ORL team, during clinical examination, secretion was found in the left middle ear. After the lack of response to clinical treatment with oral antibiotics, ventilation tube implantation was performed, followed by an investigation with computed tomography (CT) of the skull and mastoid region.
The brain CT not only revealed signs of chronic involvement of the left mastoid but also depicted a heterogeneous lesion with areas of calcification and bone destruction located in the sphenoid sinus region. This lesion projected inferiorly and laterally, invading the clivus throughout its extent, the petrous apex on the left side, the middle fossa on the same side, and extending to the pterygopalatine and infratemporal fossae on the left [
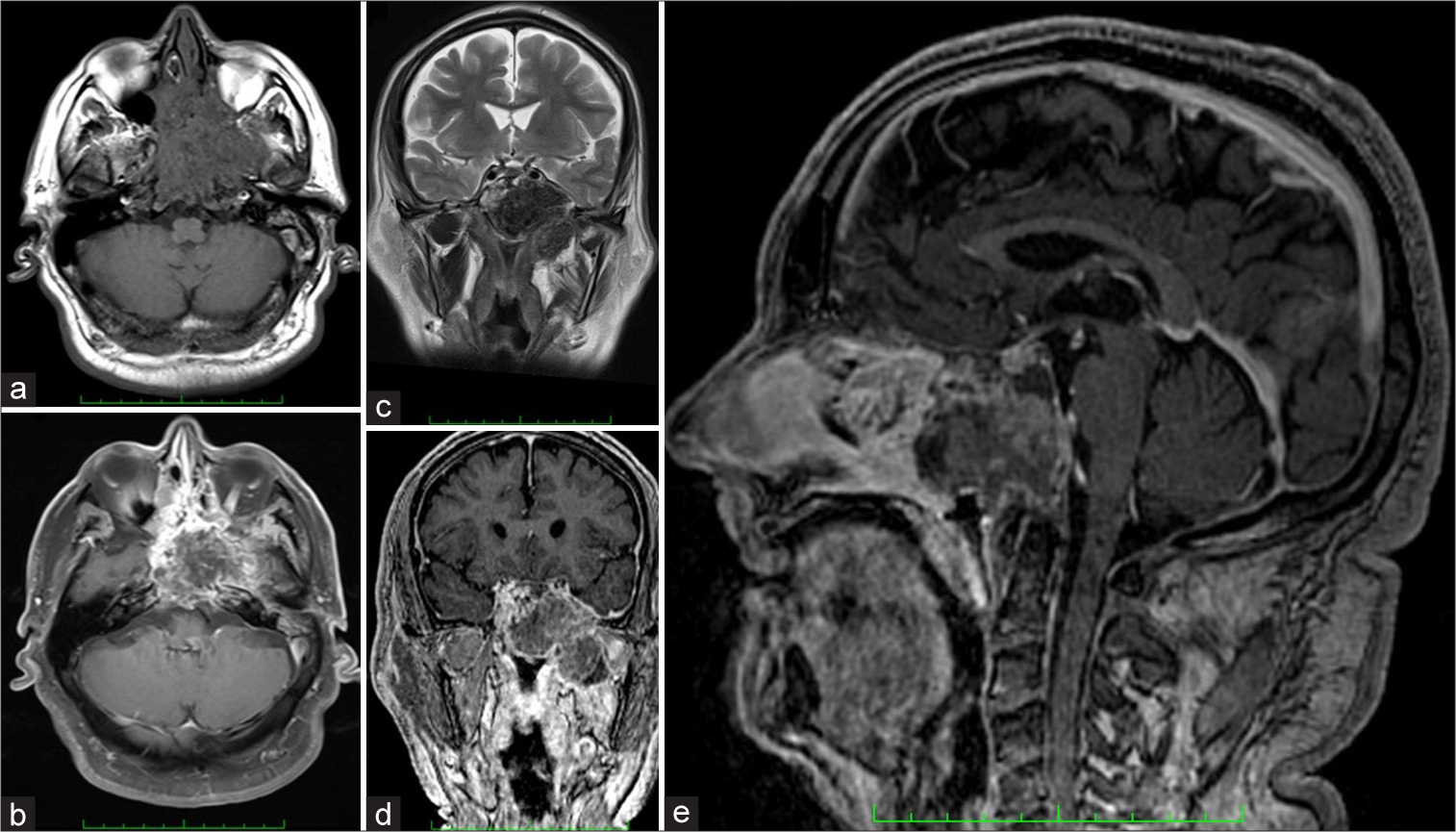
Figure 1:
Preoperative computed brain tomography showing a heterogeneous lesion in the sphenoid region with signs of calcification. In (a), an axial image showing the invasion of the clivus, petrous apex in the temporal bone. In (b), another inferior image shows the extension to the pterygopalatine and infratemporal fossae on the left. In (c) (coronal) and (d) (sagittal) images, we can see the integrity of the sellar floor and all anterior skull bases.
Figure 2:
Preoperative magnetic resonance image showing in (a and b) axial T1 without and T1 with contrasted showing a heterogenous enhancement of contrast. In (c), a coronal T2 shows a hypo signal lesion. In (d) (coronal) and (e) (sagittal) contrasted T1, showing the extension of the lesion to pterygopalatine and infratemporal fossae without invasion of the sellar floor and anterior skull base.
After MRI, the patient was referred to our multidisciplinary skull base surgery group. Following clarification of risks and benefits, surgical treatment was indicated through an extended endoscopic endonasal approach, as this provided optimal access to the tumor epicenter without jeopardizing major vessels and nerves. Meticulous planning was undertaken to ensure safe tumor removal in proximity to the internal carotid artery, particularly at its paraclival segment, considering tumor extension through the middle cranial fossa superiorly and the infratemporal fossa inferiorly. Under general anesthesia, the patient was placed in a horizontal supine position, with the head secured using Mayfield three points, and neurophysiological monitoring of cranial nerves was performed. Extended endoscopic endonasal access was performed with a transmaxillary and transpterygoid approach, followed by harvesting of a vascularized nasoseptal flap from the right side. At this point, a sizable, bleeding lesion with coarse calcifications occupying the nasal fossa was observed. We performed a central debulking of the lesion, followed by an attempt to reach all its boundaries. Subsequently, the entire anterior portion of the sellar floor was exposed to completely resect the lesion. The petrous apex was exposed without a contralateral transmaxillary corridor, allowing visualization of the genu of the petrous segment of the internal carotid artery and removal of the extradural lesion in the middle cranial fossa. Later, we addressed the pterygoid fossa region, achieving apparent complete resection. At the end of the procedure, the vascularized nasoseptal flap was positioned over the exposed left carotid, with no signs of cerebrospinal fluid leakage at any point.
The surgical pathology report revealed that it was an epithelioid neoplasm with calcifications, immunohistochemistry showed p63, cytokeratin (AE1/ AE3) and Ki-67 (<1%), and grade 1 adamantinomatous craniopharyngioma as the final diagnosis. The postoperative MRI revealed gross total resection [
DISCUSSION
Craniopharyngiomas are benign neoplasms characterized by the presence of cystic, solid, or mixed components, exhibiting irregular morphology and adherence to surrounding structures.[
The pathogenesis of infrasellar craniopharyngiomas is primarily attributed to the persistence of remnants of the craniopharyngeal duct.[
The clinical findings of these lesions are closely related to their size and location, as well as the degree of compression of surrounding structures.[
Infrasellar craniopharyngiomas share radiological characteristics with intracranial craniopharyngiomas.[
Craniopharyngiomas represent a formidable surgical challenge, even with advancements in contemporary neurosurgical techniques.[
The endoscopic approach stands out for the significant advantage of providing extensive visualization of the tumor’s origin site and the areas affected by the tumor. In comparison to the transcranial microscopic approach, the endoscopic approach holds the promise of a higher rate of total resection.[
Furthermore, recent technological advancements, such as neuronavigation and intranasal Doppler, serve as precise tools for safely addressing the skull base. The most common postoperative complication observed following the extended endoscopic endonasal approach for craniopharyngiomas is cerebrospinal fluid leakage. Other complications associated with this technique include meningitis and hydrocephalus.[
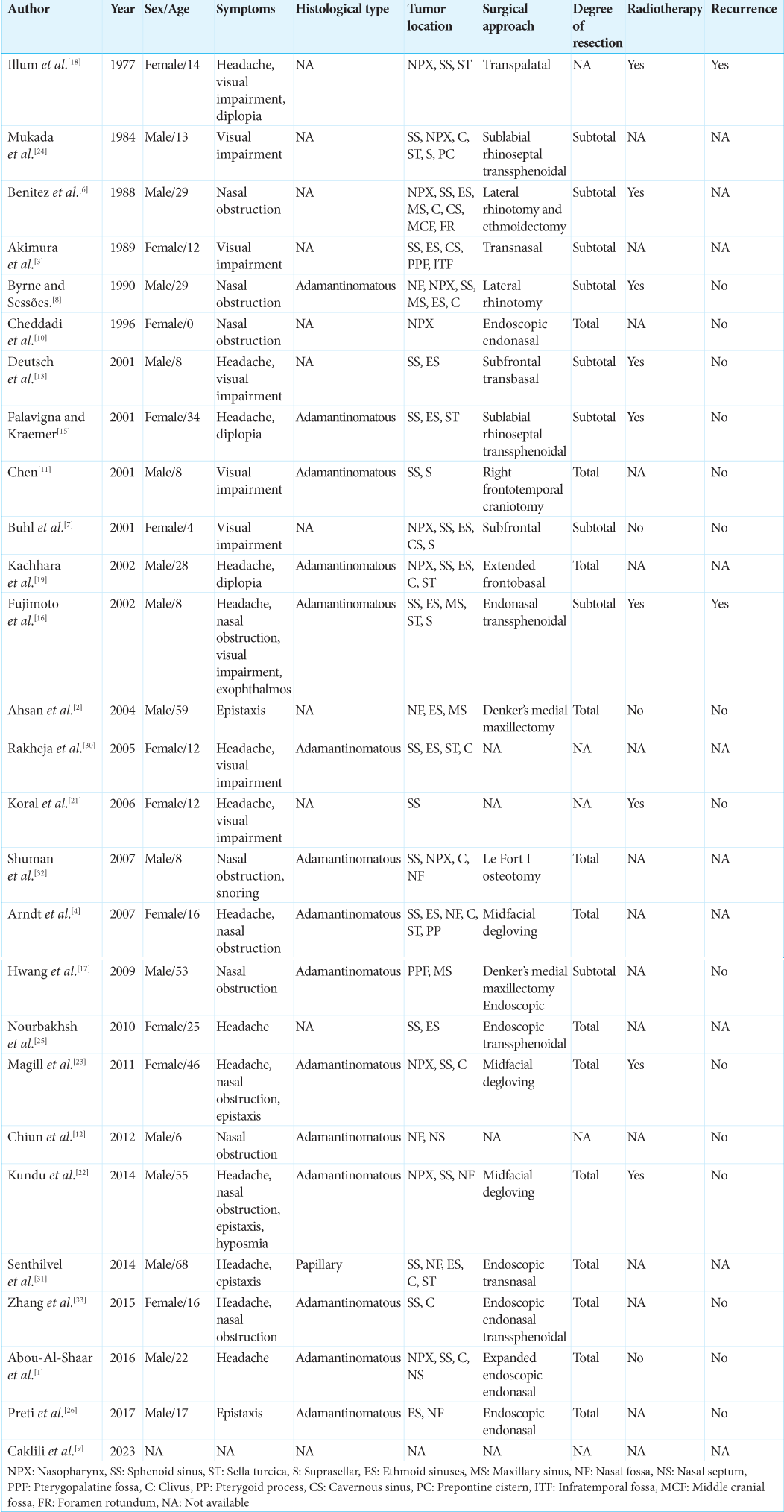
In published studies, 27 cases of infrasellar craniopharyngiomas have been documented [
CONCLUSION
We present a rare case of a giant infrasellar craniopharyngioma with extensive invasion of the skull base without involvement of sella turcica or the pituitary gland. Neuroimaging and surgical findings indicate the tumor’s origin in the sphenoidal region with subsequent invasion of the clivus, petrous apex, middle fossa, pterygopalatine, and infratemporal fossae. Although classified as benign tumors, craniopharyngiomas represent significant challenges due to their local aggressiveness, resulting in considerable morbidity. Concerns about recurrence persist even after a good degree of resection, making long-term follow-up indispensable. The extended endoscopic endonasal approach stands out for providing excellent visualization of the involved anatomy, allowing for a safe and effective approach.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abou-Al-Shaar H, Blitz AM, Rodriguez FJ, Ishii M, Gallia GL. Expanded endonasal endoscopic approach for resection of an infrasellar craniopharyngioma. World Neurosurg. 2016. 95: 618.e7-12
2. Ahsan F, Rashid H, Chapman A, Ah-See KW. Infrasellar craniopharyngioma presenting as epistaxis, excised via denker’s medial maxillectomy approach. J Laryngol Otol. 2004. 118: 895-8
3. Akimura T, Kameda H, Abiko S, Aoki H, Kido T. Infrasellar craniopharyngioma. Neuroradiology. 1989. 31: 180-3
4. Arndt S, Wiech T, Mader I, Maier W. Entire infrasellar craniopharyngioma simulating clival chordoma. Otolaryngol Head Neck Surg. 2007. 137: 981-3
5. Baldauf J, Hosemann W, Schroeder HW. Endoscopic endonasal approach for craniopharyngiomas. Neurosurg Clin N Am. 2015. 26: 363-75
6. Benitez WI, Sartor KJ, Angtuaco EJ. Craniopharyngioma presenting as a nasopharyngeal mass: CT and MR findings. J Comput Assist Tomogr. 1988. 12: 1068-72
7. Buhl R, Nabavi A, Fritsch M, Mehdorn HM. Nasopharyngeal extension of a craniopharyngioma in a 4 year old girl. Acta Neurochir (Wien). 2001. 143: 1283-5
8. Byrne MN, Sessions DG. Nasopharyngeal craniopharyngioma. Case report and literature review. Ann Otol Rhinol Laringol. 1990. 99: 633-9
9. Caklili M, Uzuner A, Yilmaz E, Duman Ozturk S, Cizmecioglu Jones FM, Balci S. Surgical outcomes and follow-up results of 53 pediatric craniopharyngioma cases: A single-center study. J Neurosurg Pediatr. 2023. 33: 223-35
10. Cheddadi D, Triki S, Gallet S, Marquet M, Muller JP, Renaud H. Neonatal rhinopharyngeal obstruction due to craniopharyngioma. Arco Pediatr. 1996. 3: 348-51
11. Chen CJ. Suprasellar and infrasellar craniopharyngioma with a persistent craniopharyngeal canal: Case report and review of the literature. Neuroradiology. 2001. 43: 760-2
12. Chiun KC, Tang IP, Vikneswaran T, Nurshaline Pauline HK. Infrasellar craniopharyngioma of the posterior nasal septum: A rare entity. Med J Malaysia. 2012. 67: 131-2
13. Deutsch H, Kothbauer K, Persky M, Epstein FJ, Jallo GI. Infrasellar craniopharyngiomas: Case report and review of the literature. Skull Base. 2001. 11: 121-8
14. Erdheim J. About hypophyseal adenomas and brain craniopharyngiomas. Akad Wiss Wien. 1904. 113: 537-726
15. Falavigna A, Kraemer JL. Infrasellar craniopharyngioma: Case report. Arq Neuropsiquiatr. 2001. 59: 424-30
16. Fujimoto Y, Matsushita H, Velasco O, Rosemberg S, Plese JP, Marino R. Craniopharyngioma involving the infrasellar region: A case report and review of the literature. Pediatr Neurosurg. 2002. 37: 210-6
17. Hwang KR, Lee JY, Byun JY, Hong HS, Koh ES. Infrasellar craniopharyngioma originating from the pterygopalatine fossa with invasion to the maxillary sinus. Br J Oral Maxillofac Surg. 2009. 47: 422-4
18. Illum P, Elbrond O, Nehen AM. Surgical treatment of nasophrayngeal craniopharyngioma. Radical removal by the transpalatal approach. J Laryngol Otol. 1977. 91: 227-33
19. Kachhara R, Nair S, Gupta AK, Radhakrishnan VV, Bhattacharya RN. Infrasellar craniopharyngioma mimicking a clival chordoma: A case report. Neurol India. 2002. 50: 198-200
20. Karavitaki N, Wass JA, editors. Craniopharyngiomas. Endocrinology and metabolism clinics of North America. Netherlands: Elsevier Saunders; 2008. 37: 173-13
21. Koral K, Weprin B, Rollins NK. Sphenoid sinus craniopharyngioma simulating mucocele. Acta Radiol. 2006. 47: 494-6
22. Kundu S, Dewan K, Varshney H, Das S, Mukhopadhyay S, Ghosh A. An atypical rare case of extracranial craniopharyngioma. Indian J Otolaryngol Head Neck Surg. 2014. 66: 122-5
23. Magill JC, Ferguson MS, Sandison A, Clarke PM. Nasal craniopharyngioma: Case report and literature review. J Laryngol Otol. 2011. 125: 517-9
24. Mukada K, Mori S, Matsumura S, Uozumi T, Goishi J. Infrasellar craniopharyngioma. Surg Neurol. 1984. 21: 565-71
25. Nourbakhsh A, Brown B, Vannemreddy P, Lian T, Nanda A, Guthikonda B. Extracranial infrasellar ectopic craniopharyngioma: A case report and review of the literature. Skull Base. 2010. 20: 475-80
26. Preti A, Karligkiotis A, Facco C, Ottini G, Volpi L, Castelnuovo P. Primary ectopic ethmoidal craniopharyngioma. J Craniofac Surg. 2017. 28: 944-6
27. Prieto R, Barrios L, Pascual JM. Strictly third ventricle craniopharyngiomas: Pathological verification, anatomo-clinical characterization and surgical results from a comprehensive overview of 245 cases. Neurosurg Rev. 2022. 45: 375-94
28. Prieto R, Juratli TA, Bander ED, Santagata S, Barrios L, Brastianos PK. Papillary Craniopharyngioma: An integrative and comprehensive review. Endocr Rev. 2024. 46: 151-213
29. Pusey E, Kortman KE, Flannigan BD, Tsuruda J, Bradley WG. MR of craniopharyngiomas: Tumor delineation and characterization. AJR Am J Roentgenol. 1987. 149: 383-8
30. Rakheja D, Meehan JJ, Gomez AM. Pathologic quiz case: Sphenoid sinus mass in a 12-year-old girl. Infrasellar adamantinomatous craniopharyngioma. Arch Pathol Lab Med. 2005. 129: e73-4
31. Senthilvel HN, Krishnan SS, Vasudevan MC. Extracranial infrasellar craniopharyngioma. Neurol India. 2014. 62: 100-3
32. Shuman AG, Heth JA, Marentette LJ, Blaivas M, Muraszko KM. Extracranial nasopharyngeal craniopharyngioma: Case report. Neurosurgery. 2007. 60: E780-1 discussion E781
33. Zhang Z, Li MY, Liu YW, Wang YZ. An infrasellar craniopharyngioma involving the sphenoid sinus and clivus. Chin Med J (Engl). 2015. 128: 844-5