- Department of Neurosurgery University of Washington, Seattle, Washington, United States.
- Department of Radiology, University of Washington, Seattle, Washington, United States.
Correspondence Address:
Zaid Aljuboori, Department of Neurosurgery, University of Washington, Seattle, Washington, United States.
DOI:10.25259/SNI_740_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zaid Aljuboori1, Margaret McGrath1, Rahul Jadhav2, Basavaraj Ghodke2, Laligam Sekhar1. A rare case of ruptured intracranial aneurysm arising from the retro-mastoid branch of the occipital artery. 13-Sep-2021;12:458
How to cite this URL: Zaid Aljuboori1, Margaret McGrath1, Rahul Jadhav2, Basavaraj Ghodke2, Laligam Sekhar1. A rare case of ruptured intracranial aneurysm arising from the retro-mastoid branch of the occipital artery. 13-Sep-2021;12:458. Available from: https://surgicalneurologyint.com/surgicalint-articles/11112/
Abstract
Background: Aneurysms of the occipital artery (OA) are rare, with few cases published in the literature. The pathophysiology is unknown, and the presentation is variable. We present a case of a ruptured intracranial aneurysm arising from a branch of the OA.
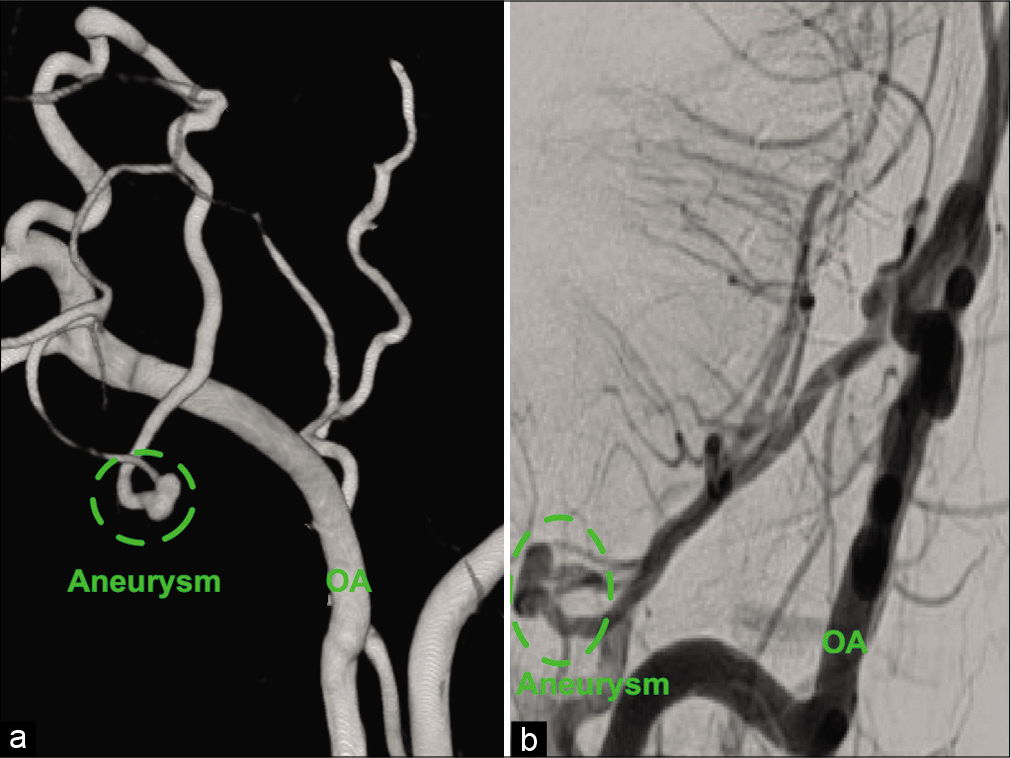
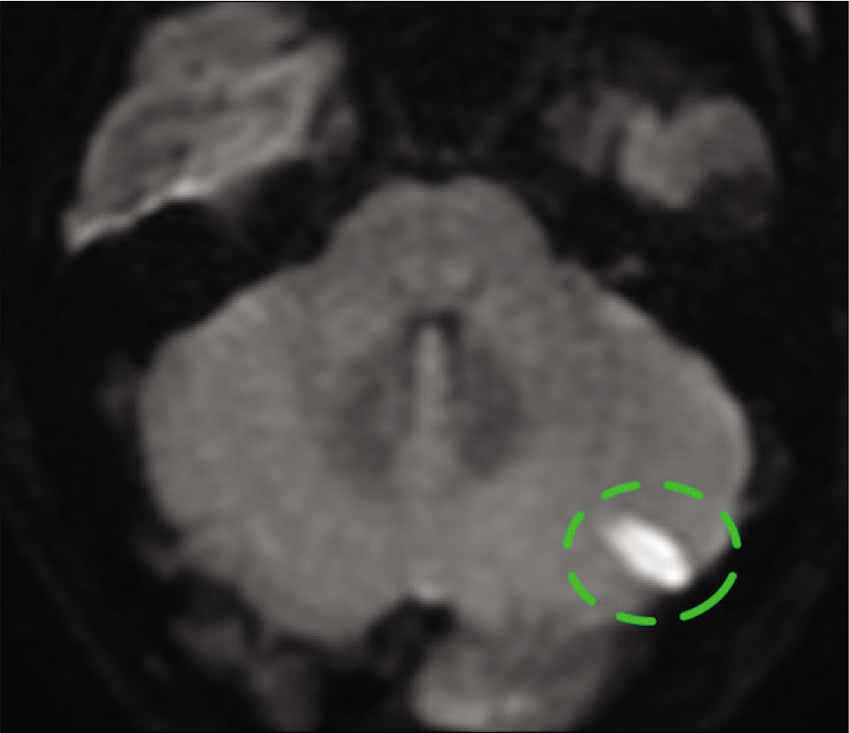
Case Description: A 36-year-old male with a history of ankylosing spondylitis presented with altered mental status after an assaulted. On examination, he was intubated, with a Glasgow coma scale of 9, and imaging of the head and neck revealed a subdural hematoma of the posterior fossa and the cervical spine. The patient underwent suboccipital craniectomy and C1-5 laminectomy with the evacuation of the subdural hematoma. Postoperative cerebral angiography showed an intracranial aneurysm arising from the retromastoid branch of the OA on the left side. Furthermore, the parent vessel of the aneurysm supplied the left lower half of the cerebellar hemisphere. The aneurysm and the parent vessel were embolized using platinum coils. The patient tolerated the procedure well, and magnetic resonance imaging of the brain showed a minor left-sided cerebellar infarct, which was asymptomatic. The patient was discharged home with a modified Rankin scale of 2. There were no outpatient follow-up data available because the patient lost to follow-up.
Conclusion: Intracranial OA aneurysms are extremely rare with no clear consensus concerning the management of these aneurysms. They can be treated using endovascular and or open surgical techniques depending on the aneurysm characteristics, patient condition, rupture status, and others.
Keywords: Aneurysm, Artery, Occipital, Posterior inferior cerebellar artery
INTRODUCTION
Aneurysms of the occipital artery (OA) can be true or pseudoaneurysms. The true aneurysm subtype is rare, with only ten cases published in the literature.[
CASE DESCRIPTION
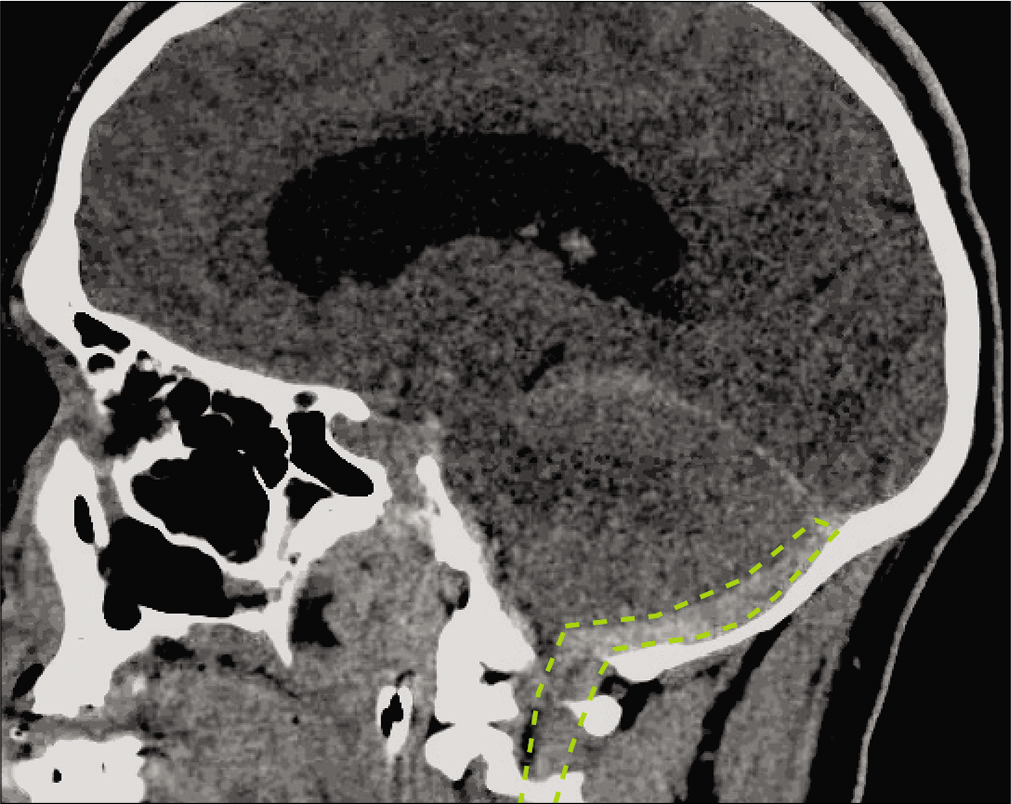
The patient is a 36-year-old male with a history of ankylosing spondylitis, COPD, and seizure disorder presented with altered mental status after being assaulted. On exam, he was intubated, with a Glasgow coma scale of 9, and imaging of the head and neck revealed a subdural hematoma of the posterior fossa and the cervical spine [
Figure 3:
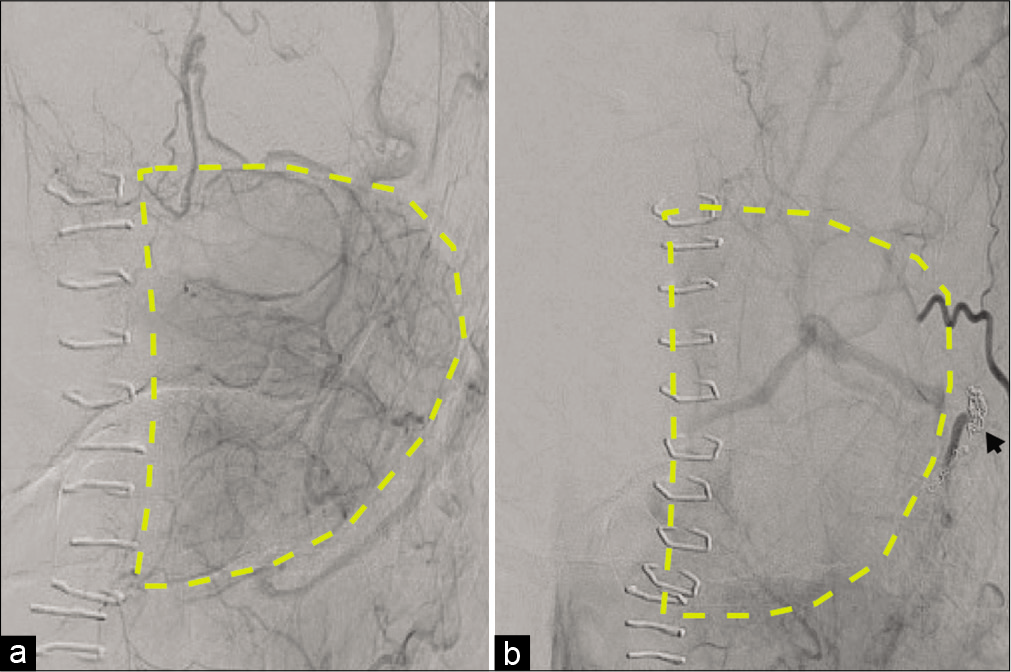
(a) A subtracted angiographic image (pre-embolization) of the left occipital artery (capillary phase) that shows the cerebellar blood supply. (b) A subtracted angiographic image (post-embolization) of the left occipital artery (capillary phase) that shows the diminished cerebellar blood supply.
Figure 4:
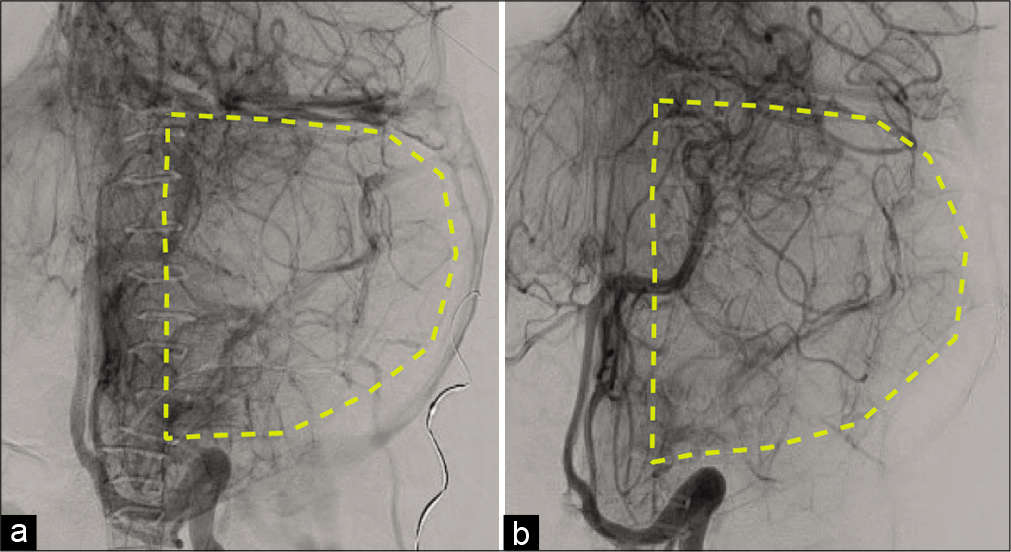
(a) A subtracted angiographic image (pre-embolization) of the left posterior inferior cerebellar artery (late-arterial phase) that shows the cerebellar blood supply. (b) A subtracted angiographic image (post-embolization) of the left occipital artery (late-arterial phase) that shows the increased cerebellar blood supply.
DISCUSSION
True aneurysms of the OA are rare, with few cases reported in the literature.[
The management of OA aneurysms includes observation or obliteration using endovascular or open surgical techniques. The management strategy should consider the aneurysm type (true or pseudo-aneurysm), location, size, rupture status, vascular anatomy, and patient condition. Our review of the literature showed that extracranial OA aneurysms are more likely to be treated using endovascular embolization or trapping with excision.[
In our case, we decided to embolize the aneurysm and the parent vessel because of the aneurysm location and morphology, which precluded preserving the parent vessel. Furthermore, the balloon test occlusion of the OA showed cross filling of the left lower cerebellar hemisphere through the PICA, which indicated that the likelihood of developing a fulminant cerebellar stroke is exceptionally low.
Although rare, OA aneurysms should be considered in cases of subdural hematoma of the posterior fossa, pulsatile scalp mass or neck mass, or nontraumatic neck hematoma. Both endovascular and open surgical techniques are effective for treating these aneurysms.
CONCLUSION
Intracranial OA aneurysms are extremely rare and frequently present with subdural hematoma. The etiology remains elusive, but there appears to be an association with increased flow demand. The management of these aneurysms includes both endovascular and or open surgical techniques and depends on several factors such as the aneurysm characteristics, patient condition, rupture status, and others.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bissacco D, Domanin M, Romagnoli S, Martelli E, Civelli V, Gabrielli L. Spontaneous rupture of multiple occipital artery aneurysms in a patient with neurofibromatosis Type 1. Vasc Endovasc Surg. 2018. 52: 86-8
2. Chaudhry NS, Gaynor BG, Hussain S, Dernbach PD, Aziz-Sultan MA. Etiology and treatment modalities of occipital artery aneurysms. World Neurosurg. 2017. 102: 697.e1-4
3. Gum GK, Numaguchi Y, Nadell JM, Robinson AE. Aneurysm of the occipital artery: Development after surgical ligation of the internal carotid artery. AJNR Am J Neuroradiol. 1987. 8: 1155-6
4. Illuminati G, Cannistra M, Pizzardi G, Pasqua R, Frezzotti F, Calio FG. True aneurysm of the proximal occipital artery: Case report. Int J Surg Case Reports. 2018. 43: 32-5
5. Kanematsu M, Kato H, Kondo H, Goshima S, Tsuge Y, Kojima T. Neurofibromatosis Type 1: Transcatheter arterial embolization for ruptured occipital arterial aneurysms. Cardiovasc Intervent Radiol. 2011. 34: S131-5
6. Kawasaki T, Yoshida K, Kikuchi T, Ishii A, Takagi Y, Miyamoto S. Ruptured aneurysms of the occipital artery associated with congenital occipital bone defect. World Neurosurg. 2017. 97: 759.e13-5
7. Kim HS, Son BC, Lee SW, Kim IS. A rare case of spontaneous true aneurysm of the occipital artery. J Korean Neurosurg Soc. 2010. 47: 310-2
8. Lafford G, Sharp O, Rosich-Medina A. True occipital artery aneurysm: A surgical case report and literature review. JPRAS Open. 2020. 25: 40-5
9. Nguyen HS, Doan N, Shabani S, Gelsomino M, Zaidat O. Pure tentorial subdural hematoma from rupture of aneurysm along the transmastoid branches of the occipital artery. Surg Neurol Int. 2016. 7: S499-501
10. Sloniewski P, Dzierzanowski J, Och W. Aneurysm of the meningeal branch of the occipital artery connecting with the distal portion of the posteroinferior cerebellar artery by the dural fistula. Folia Morphol (Warsz). 2008. 67: 292-5