- John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu, Hawaii, United States
- Department of Neurosurgery, The Queen’s Medical Center, Honolulu, Hawaii, United States
- Department of Pathology, The Queen’s Medical Center, Honolulu, Hawaii, United States
- Department of Neurointerventional Surgery, The Queen’s Medical Center, Honolulu, Hawaii, United States
- Department of Neuroscience Institute, The Queen’s Medical Center, Honolulu, Hawaii, United States.
Correspondence Address:
Joo Won Choi, John A. Burns School of Medicine, University of Hawaii at Manoa, Honolulu, Hawaii, United States.
DOI:10.25259/SNI_733_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Joo Won Choi1, Richard Ho1, Yi Jonathan Zhang2, Wichit Sae-Ow3, Ferdinand K. Hui4, Stacy C. Brown5, Samuel Tsappidi4. A rare case of solitary, isolated dural metastasis from hepatocellular carcinoma mimicking a meningioma. 10-Nov-2023;14:398
How to cite this URL: Joo Won Choi1, Richard Ho1, Yi Jonathan Zhang2, Wichit Sae-Ow3, Ferdinand K. Hui4, Stacy C. Brown5, Samuel Tsappidi4. A rare case of solitary, isolated dural metastasis from hepatocellular carcinoma mimicking a meningioma. 10-Nov-2023;14:398. Available from: https://surgicalneurologyint.com/surgicalint-articles/12628/
Abstract
Background: Distinguishing an isolated metastatic dural tumor from a meningioma on imaging is challenging and may lead to a delay in treatment. Here, we present the first known case of isolated, solitary dural metastasis from hepatocellular carcinoma (HCC) mimicking a meningioma.
Case Description: A 64-year-old male with a history of liver cirrhosis presented with a 5.8 cm enhancing left parafalcine hemorrhagic dural-based mass extending across the midline. Cerebral angiography revealed a distal left anterior pseudoaneurysm, and tumor contrast blush with feeders from the left ophthalmic and right middle meningeal artery. The pseudoaneurysm was successfully embolized to stop the bleeding, followed by an uneventful bi-coronal frontal craniotomy for falcine tumor resection to relieve brain compression. Histopathological analysis of the dural-based tumor showed poorly differentiated carcinoma with positive albumin in situ hybridization and cytokeratin tumor markers, consistent with dural metastases from HCC.
Conclusion: When encountering a solitary, highly vascular mass bearing resemblance to a meningioma, it may be prudent to consider the possibility of a dural-based metastatic carcinoma.
Keywords: Dural metastasis, Hepatocellular carcinoma, Meningioma
INTRODUCTION
With advancements in systemic treatments that have prolonged survival times in patients with late-stage malignancies, there has been a growing body of literature demonstrating cancer metastases to previously uncommon sites. Among them include metastases to the brain dura mater, which are rare, late manifestations of solid tumors that often portend poor prognoses.[
To date, reported cases of isolated dural metastasis have been limited to patients with primary malignancies of the lung, breast, or prostate; dural extension from hepatocellular carcinoma (HCC) has yet to be described.[
CASE DESCRIPTION
A 64-year-old male presented to the emergency department from his care home with 12 days of dysarthria and right hemiparesis and three days of progressively worsening altered mental status. His medical history was significant for homelessness, chronic hepatitis C, liver cirrhosis, polysubstance use disorder, and schizoaffective disorder. Of note, an incidental abdominal and pelvic computed tomography (CT) scan for a urinary tract infection obtained one year before admission was negative for any liver masses.
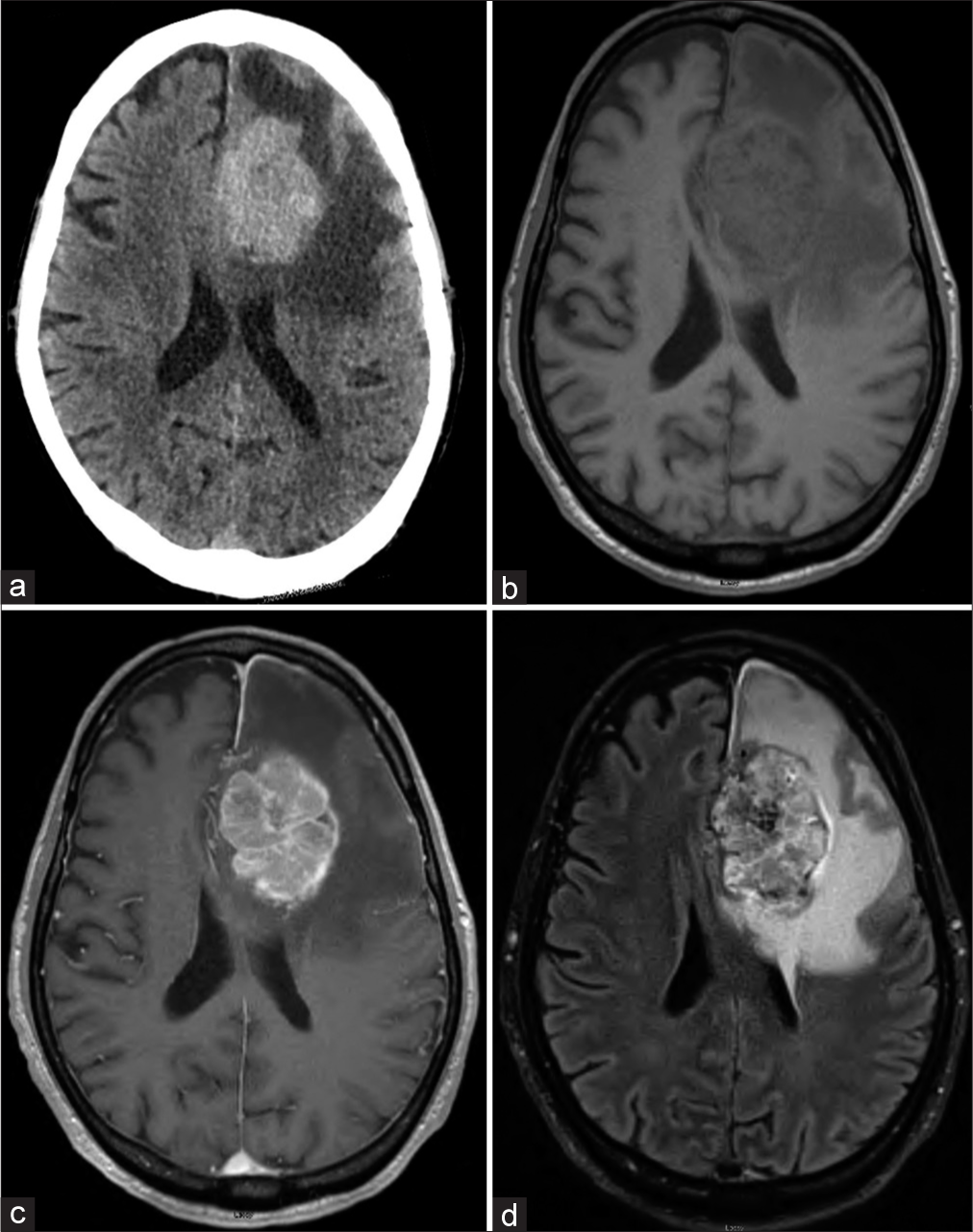
The patient’s initial examination was notable for the left gaze preference, global aphasia, and right hemiparesis, with head CT showing a large hypervascular 5.7 cm tumor with surrounding edema [
Figure 1:
(a) Initial head CT showing a 5.7 cm left frontal hypervascular tumor with significant edema. (b) Pre- and (c) post-contrast T1-weighted MRI showing a 5.8 cm enhancing extra-axial left parafalcine hemorrhagic mass crossing midline. (d) T2-FLAIR MRI shows extensive surrounding vasogenic edema. CT: Computed tomography, MRI: Magnetic resonance imaging, FLAIR: Fluid-attenuated inversion recovery.
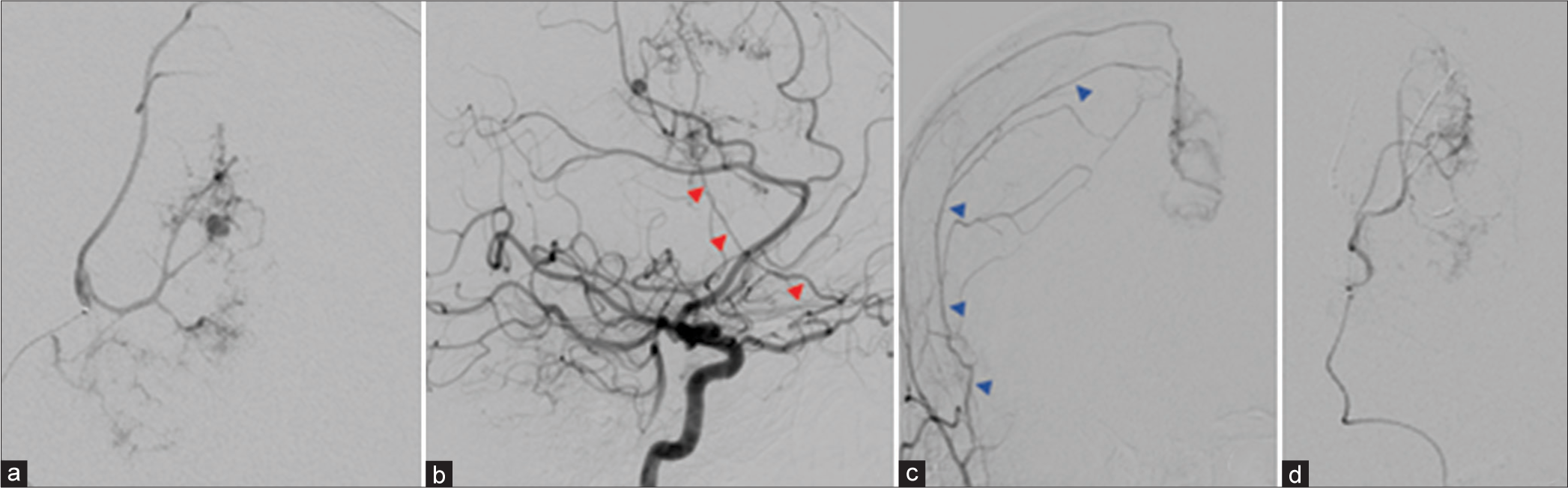
Figure 2:
Cerebral angiography showing (a) left ACA pseudoaneurysm, and tumor contrast blush with additional supply from the (b) left meningeal branch of the left ophthalmic artery (red arrows) and the (c) right middle meningeal artery (blue arrows). (d) Post-embolization of the ACA pseudoaneurysm. ACA: Anterior cerebral artery.
The patient’s postoperative course was complicated by persistent neurologic disability, Klebsiella pneumoniae and Methicillin-sensitive Staphylococcus aureus pneumonia, and prolonged ventilator dependence. Given his advanced disease, the patient was transitioned to comfort measures following a discussion with the family and expired on day 18 of hospitalization. Histopathological findings of the dural-based tumor returned following the patient’s passing, revealing a poorly differentiated carcinoma with positive epithelial markers and in situ hybridization staining for albumin [
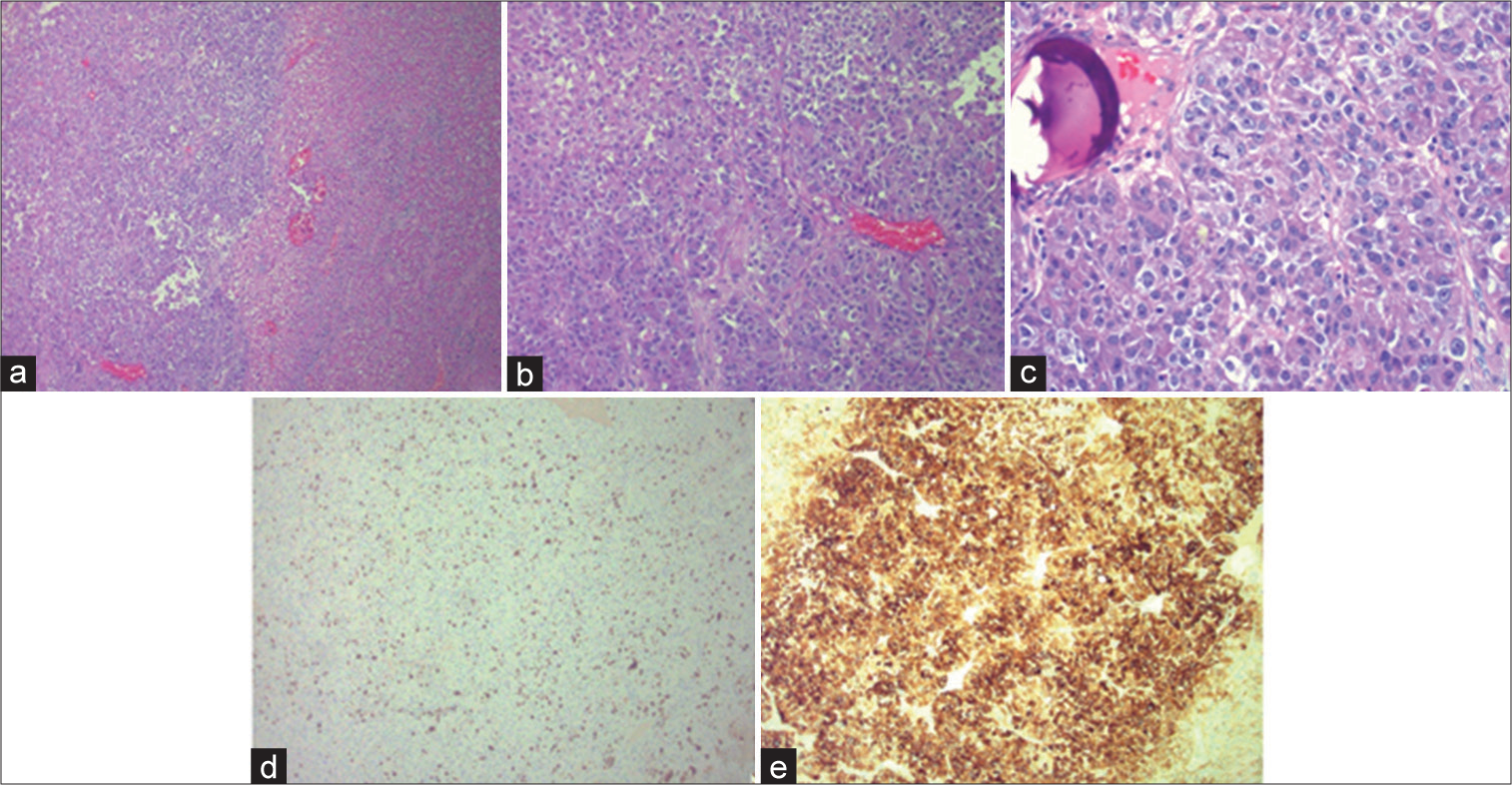
Figure 4:
Prepared slides of the dural-based tumor stained with hematoxylin and eosin showing (a) viable tumor (left) and necrotic tumor (right), (b) cohesive nests of tumor cells with a fair amount of eosinophilic cytoplasm, and (c) atypical tumor cells with brisk mitotic activity, nuclear pleomorphism, and prominent nucleoli. Immunohistochemical examination showing (d) markedly elevated ki-67 index of 25%, supporting the malignant nature of the tumor. (e) The tumor was diffusely positive for epithelial markers (cytokeratin AE1/AE3) and negative for hepatocellular markers (HepPar1, glypican 3), though albumin in situ hybridization was positive in a large subset of tumor cells. These findings in a patient with a history of liver cirrhosis were consistent with metastatic hepatocellular carcinoma.
DISCUSSION
Intra- and extra-axial brain metastasis are very rare manifestations of HCC, with an estimated incidence of 0.3–2.2%.[
Several hypothesized mechanisms detail how tumor seeding of the dura mater may occur. One such route involves direct extension from existing calvarial metastases to the dura, most commonly in breast and prostate cancer.[
Diagnosis of dural metastatic carcinoma is challenging as they are both clinically and radiographically difficult to distinguish from meningiomas.[
CONCLUSION
Solitary, isolated metastasis to the dura mater is a rare manifestation of HCC that is seldom the initial clinical presentation and is imperative to distinguish from a meningioma. When faced with an isolated, hypervascular mass resembling a meningioma, a metastatic tumor should remain on the differential.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH. Brain metastases from hepatocellular carcinoma: Prognostic factors and outcome: Brain metastasis from HCC. J Neurooncol. 2009. 91: 307-13
2. Erdogan H, Aydin MV, Tasdemiroglu E. Tumor-to-tumor metastasis of the central nervous system. Turk Neurosurg. 2014. 24: 151-62
3. Harrison RA, Nam JY, Weathers SP, DeMonte F. Intracranial dural, calvarial, and skull base metastases. Handb Clin Neurol. 2018. 149: 205-25
4. Higuchi M, Fujimoto Y, Miyahara E, Ikeda H. Isolated dural metastasis from colon cancer. Clin Neurol Neurosurg. 1997. 99: 135-7
5. Kleinschmidt-DeMasters BK. Dural metastases. A retrospective surgical and autopsy series. Arch Pathol Lab Med. 2001. 125: 880-7
6. Kwon H, Kim JW, Park M, Kim JW, Kim M, Suh SH. Brain metastases from lung adenocarcinoma may preferentially involve the distal middle cerebral artery territory and cerebellum. Front Oncol. 2020. 10: 1664
7. Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005. 75: 57-61
8. Lui YW, Malhotra A, Farinhas JM, Dasari SB, Weidenheim K, Freeman K. Dynamic perfusion MRI characteristics of dural metastases and meningiomas: A pilot study characterizing the first-pass wash-in phase beyond relative cerebral blood volume. AJR Am J Roentgenol. 2011. 196: 886-90
9. Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer. 2009. 115: 1947-53
10. Sartori Balbinot R, Facco Muscope AL, Dal Castel M, Sartori Balbinot S, Angelo Balbinot R, Soldera J. Intraparenchymal hemorrhage due to brain metastasis of hepatocellular carcinoma. Case Rep Gastroenterol. 2017. 11: 516-25
11. Savage NM, Alleyne CH, Vender JR, Figueroa R, Zhang H, Samuel TA. Dural-based metastatic carcinomas mimicking primary CNS neoplasia: Report of 7 cases emphasizing the role of timely surgery and accurate pathologic evaluation. Int J Clin Exp Pathol. 2011. 4: 530-40
12. Shah A, Choudhri O, Jung H, Li G. Preoperative endovascular embolization of meningiomas: Update on therapeutic options. Neurosurg Focus. 2015. 38: E7
13. Sidani C, Rodriguez JR, Rahal ON. Solitary dural metastasis from renal cell carcinoma mimicking meningioma nine years after complete remission: Case report and a literature review. J Clin Case Rep. 2018. 8: 1144
14. Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007. 13: 414-20
15. Zhao C, Wei Y, Liu J, Xu S, Jiang X, Di G. Spontaneous acute epidural hematoma associated with chronic subdural hematoma due to dural metastasis of gastric carcinoma: A case report and literature review. Int J Neurosci. 2021. 131: 405-10