- George Washington School of Medicine and Health Sciences, Washington, United States
- Department of Neurological Surgery, George Washington School of Medicine and Health Sciences, Washington, United States
Correspondence Address:
Jayanidhi Kedda, Department of Neurological Surgery, George Washington School of Medicine and Health Sciences, Washington, United States.
DOI:10.25259/SNI_402_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Saba Rentia1, Jayanidhi Kedda2, Peter Harris2, Chase Harrison Foster2, Michael Rosner2. A rare case of Streptobacillus moniliformis epidural abscess requiring neurosurgical decompression. 26-Jul-2024;15:263
How to cite this URL: Saba Rentia1, Jayanidhi Kedda2, Peter Harris2, Chase Harrison Foster2, Michael Rosner2. A rare case of Streptobacillus moniliformis epidural abscess requiring neurosurgical decompression. 26-Jul-2024;15:263. Available from: https://surgicalneurologyint.com/surgicalint-articles/13007/
Abstract
Background: Streptobacillus moniliformis is the primary causative agent of rat bite fever, an infectious disease transmitted through contact with rats through bites, scratches, or exposure to excrement. Before this report, only two instances of spinal epidural abscess (SEA) due to S. moniliformis infection have been documented. We present the case of a 76-year-old male who developed a cervical SEA secondary to S. moniliformis infection, requiring neurosurgical decompression of the spinal cord.
Case Description: A 76-year-old male presented to the emergency department with bilateral shoulder and back pain, upper extremity weakness, left hip pain, and left thumb pain. He denied any recent exposure to pets or animals, and the initial workup did not yield the source of the infection. Enhanced magnetic resonance imaging of the cervical spine demonstrated C6–7 discitis/osteomyelitis and an associated ventral SEA, as well as discitis/osteomyelitis of the C2 vertebral body and C5–6 endplates. Subsequently, the patient underwent a C3–7 laminectomy and received a 6-week postoperative course of intravenous ceftriaxone, resulting in complete resolution of the abscess. Blood tests revealed the presence of S. moniliformis, which the patient attributed to potential rat exposure at his workplace.
Conclusion: Identification and diagnosis of S. moniliformis infection requires a high index of suspicion. Neurosurgeons should consider this rare pathogen in the differential diagnosis of SEA to facilitate early detection, diagnosis, and surgical intervention, ultimately improving patient outcomes.
Keywords: Cervical spinal epidural abscess, Rat bite fever, Spinal decompression, Streptobacillus moniliformis
INTRODUCTION
Rat bite fever (RBF), caused by the Streptobacillus moniliformis bacterium, is an uncommon infectious disease pathogen commonly transmitted through exposure to and accidental consumption of rodent droppings. Less common still are cases of S. moniliformis spinal epidural abscesses (SEA); only two cases have been previously reported, one in the thoracolumbar spine and another in the lumbar spine. Herein, we describe a cervical RBF SEA requiring neurosurgical intervention, the first reported in the literature to our knowledge.
CASE REPORT
A 76-year-old male with no significant medical history presented through the emergency department (ED) with new-onset bilateral shoulder and back pain. The patient described himself as a healthy individual at baseline. He was an active runner and had not had reason to see a physician in years. He reported that the pain started in his right shoulder about 1 week before admission and spread to his upper back and left shoulder. These pains continued to progress throughout the week despite over-the-counter non-steroidal anti-inflammatories. Eventually, he began to develop indolent weakness; by the time of evaluation, he was unable to lift any objects over his head. He then noticed left hip pain that made ambulation difficult as well as new left thumb pain, prompting him to present through the ED. He denied sick contacts, recent travel, outdoor expeditions, recent unclean or public water exposures, and pet or animal exposures. He was subsequently admitted to the medicine service for a diagnostic workup.
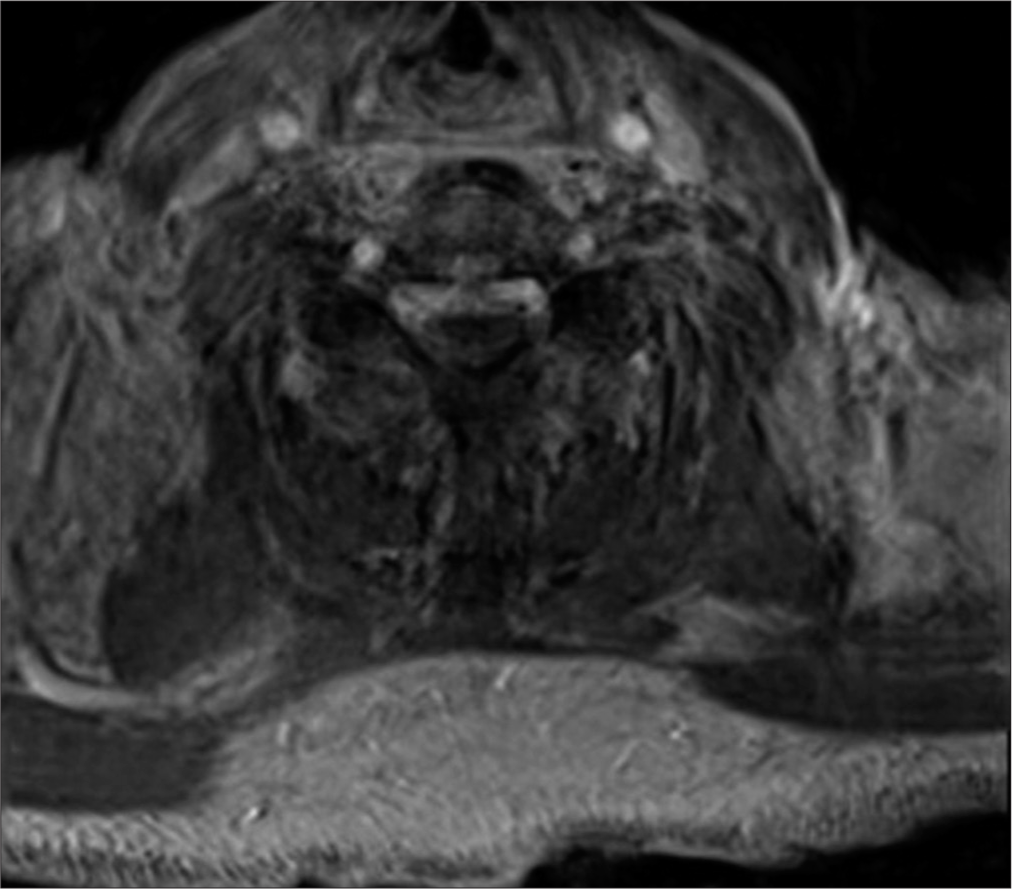
The patient was afebrile and hemodynamically stable on room air. He had a cryptogenic leukocytosis with elevated inflammatory markers (C-reactive protein of 353 and erythrocyte sedimentation rate of 87) and a concomitant acute kidney injury. A urinalysis, chest, hip, and shoulder X-rays, blood cultures, and trans-thoracic echocardiogram did not elucidate a source of infection. Non-contrast head computed tomography (CT) was normal. The rheumatology service was, thus, consulted and listed their primary differential diagnosis as polymyalgia rheumatica; however, they noted irreconcilability between this and his worsening infectious stigmata. This triggered further advanced imaging workup. A whole-body positron emission tomography/CT scan showed hypermetabolism of the right greater than the left shoulder, left hip joint, left thumb, and lateral wrist, suggestive of multifocal arthritis and myositis. Gadolinium-enhanced magnetic resonance imaging (MRI) brain demonstrated small foci of restricted diffusion in the subcortical left cerebellar and left posterior parietal lobes with corresponding T2/fluid-attenuated inversion recovery hyperintensity most consistent with subacute embolic infarcts. Enhanced MRI of his cervical spine, however, revealed C6–7 discitis/osteomyelitis and an associated 2.8 cm ventral SEA, as well as discitis/osteomyelitis of the C2 vertebral body and C5–6 endplates, for which the neurosurgery team was ultimately consulted [
Neurosurgical evaluation and intervention
On examination, the patient’s mental status and cranial nerves were intact. He had pain-limited weakness in the bilateral deltoids, objective weakness in the distal left upper extremity and left lower extremity, and positive Babinski signs bilaterally. Given these clinical and corresponding radiographic findings, we recommended multilevel cervical laminectomy for decompression of his spinal cord and the acquisition of samples to guide antibiosis.
On hospital day 5, laminectomies of C3 through C7 were performed using the ultrasonic bone scalpel, as is our institutional norm. Intraoperative ultrasound confirmed adequate dorsal decompression despite the residual ventral collection. Shortly after laminectomy, the patient had improved signals per intraoperative neuromonitoring in bilateral deltoid motor-evoked potentials, stable somatosensory-evoked potentials, and low amplitude improvement in the bilateral lower extremities. The “lobster-tailed” lamina was sent for culture; no definitive infection was identified. The spinal column was left un-instrumented, given the concern for active infection. A suprafascial wound vacuum was placed to allow for healing with the secondary intention of avoiding the high risk of subsequent wound breakdown.
Postoperative course
The patient returned to the ward where, over the coming days, he noted increased left deltoid and hip flexor strength. His blood was sent for Karius testing™ as recommended by the infectious disease team on hospital day 5. This testing yielded a result of S. moniliformis 1 week later, an organism associated with RBF. When this came to light and on further questioning, the patient noted that while he did not have any recollection of direct rat encounters, he was aware that one or more were occupying the vents at his place of employment. That same day, a transesophageal echocardiogram demonstrated centimetersized vegetations on the posterior mitral valve leaflet which was felt to be the likely source of the multifocal central nervous system emboli and seeding. The infectious disease team recommended 2 g of intravenous (IV) ceftriaxone daily for 6 weeks. The patient deferred cardiac intervention for the demonstrated vegetation. The patient was discharged home safely on hospital day 19. He was instructed to follow up in our neurosurgery clinic but was unfortunately lost to follow-up.
DISCUSSION
S. moniliformis is a highly pleomorphic bacterium, appearing as a filamentous, Gram-negative, nonmotile, and non-acid-fast rod.[
Rats are considered the primary natural reservoir of S. moniliformis, but guinea pigs, gerbils, ferrets, and squirrels can also carry it.[
RBF is a systemic illness that can present acutely or follow a relapsing remitting course.[
Given the nonspecific presentation of RBF and difficulties associated with the isolation and identification of S. moniliformis, diagnosis of the illness requires a high index of suspicion. Growth of S. moniliformis requires microaerophilic conditions and a specific culture medium enriched with 10–30% blood or serum.[
To date, there have only been two previous reports of patients with an epidural abscess caused by S. moniliformis; neither of these have involved the cervical spine. In 2012, a 58-year-old male presenting with a 2-week history of right-sided flank pain, fevers, and lower extremity weakness was found to have an abscess located between the L4 and S1 vertebrae.[
Although SEA predominantly affects the lumbar or thoracic spine, epidural abscess in the cervical spine has been identified in 18–36% of cases.[
CONCLUSION
S. moniliformis is an incredibly uncommon pathological organism, especially as a cause of SEA. Our case is only the third in the neurosurgical body of literature to describe a patient with an epidural collection requiring neurosurgical decompression. Neurosurgeons should consider this rare entity in the differential diagnosis of SEA, and further representation in the neurosurgical literature may help improve early suspicion, identification, and surgical management of this morbid and potentially mortal disease. A cohesive multidisciplinary team of hospitalists, infectious disease experts, and neurosurgeons remains critical to optimizing outcomes in these rare and challenging cases.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adams SH, Mahapatra R. Rat bite fever with osteomyelitis and discitis: Case report and literature review. BMC Infect Dis. 2021. 21: 479
2. Addidle M, Pynn J, Grimwade K, Giola M. Epidural abscess caused by Streptobacillus moniliformis. J Clin Microbiol. 2012. 50: 3122-4
3. Akter R, Boland P, Daley P, Rahman P, Al Ghanim N. Rat bite fever resembling rheumatoid arthritis. Can J Infect Dis Med Microbiol. 2016. 2016: 7270413
4. Crofton KR, Ye J, Lesho EP. Severe recurrent Streptobacillus moniliformis endocarditis in a pregnant woman, and review of the literature. Antimicrob Resist Infect Control. 2020. 9: 119
5. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007. 20: 13-22
6. Ghobrial GM, Viereck MJ, Margiotta PJ, Beygi S, Maulucci CM, Heller JE. Surgical management in 40 consecutive patients with cervical spinal epidural abscesses. Spine (Phila Pa 1976). 2015. 40: E949-53
7. Gupta M, Bhansali RK, Nagalli S, Oliver TI, editors. Rat-bite fever. StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. p.
8. Hammer A, Wolff D, Geißdörfer W, Schrey M, Ziegler R, Steiner HH. A spinal epidural abscess due to Streptobacillus moniliformis infection following a rat bite: Case report. J Neurosurg Spine. 2017. 27: 92-6
9. Hirschhorn RB, Hodge RR. Identification of risk factors in rat bite incidents involving humans. Pediatrics. 1999. 104: e35
10. Lambe DW, McPhedran AM, Mertz JA, Stewart P. Streptobacillus moniliformis isolated from a case of Haverhill fever: Biochemical characterization and inhibitory effect of sodium polyanethol sulfonate. Am J Clin Pathol. 1973. 60: 854-60
11. Loridant S, Jaffar-Bandjee MC, La Scola B. Shell vial cell culture as a tool for Streptobacillus moniliformis “resuscitation”. Am J Trop Med Hyg. 2011. 84: 306-7
12. Papadakis SA, Ampadiotaki MM, Pallis D, Tsivelekas K, Nikolakakos P, Agapitou L. Cervical spinal epidural abscess: Diagnosis, treatment, and outcomes: A case series and a literature review. J Clin Med. 2023. 12: 4509
13. Rodino KG, Miller NE, Pethan KD, DeSimone DC, Schuetz AN. The brief case: Rat bite fever from a kiss. J Clin Microbiol. 2019. 58: e00677-19
14. Schachter ME, Wilcox L, Rau N, Yamamura D, Brown S, Lee CH. Rat-bite fever, Canada. Emerg Infect Dis. 2006. 12: 1301-2
15. Smallbones M, Monem M, Baganeanu M, Okocha M, Sofat R. Near-fatal periprosthetic infection with Streptobacillus moniliformis Case and review. J Bone Jt Infect. 2020. 5: 50-3
16. Stricsek G, Iorio J, Mosley Y, Prasad S, Heller J, Jallo J. Etiology and surgical management of cervical spinal epidural abscess (SEA): A systematic review. Global Spine J. 2018. 8: 59S-67
17. Turner A, Zhao L, Gauthier P, Chen S, Roffey DM, Wai EK. Management of cervical spine epidural abscess: A systematic review. Ther Adv Infect Dis. 2019. 6: 204993611986394
18. Wegner AM, Look N, Haus BM. Surgical management of multijoint septic arthritis due to rat-bite fever in a pediatric patient: A case study. Case Rep Orthop. 2017. 2017: 2183941
19. Wullenweber M. Streptobacillus moniliformisa zoonotic pathogen, Taxonomic considerations, host species, diagnosis, therapy, geographical distribution. Lab Anim. 1995. 29: 1-15
20. Zhang WW, Hu YB, He GX, Zhou Y, Hong L, Ding JG. Rat bite fever caused by Streptobacillus moniliformis infection in a Chinese patient. BMC Infect Dis. 2019. 19: 637