- Department of Neurosurgery, The University of Tokyo, Bunkyo-ku, Japan
- Department of Radiology, The University of Tokyo, Bunkyo-ku, Japan
Correspondence Address:
Motoyuki Umekawa, Department of Neurosurgery, The University of Tokyo, Bunkyo-ku, Japan.
DOI:10.25259/SNI_443_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masayuki Nakamura1, Motoyuki Umekawa1, Yuki Shinya1, Hirotaka Hasegawa1, Atsuto Katano2, Nobuhito Saito1. A single-session stereotactic radiosurgery for vagal paraganglioma: Effective tumor reduction and innovative treatment option. 30-Aug-2024;15:314
How to cite this URL: Masayuki Nakamura1, Motoyuki Umekawa1, Yuki Shinya1, Hirotaka Hasegawa1, Atsuto Katano2, Nobuhito Saito1. A single-session stereotactic radiosurgery for vagal paraganglioma: Effective tumor reduction and innovative treatment option. 30-Aug-2024;15:314. Available from: https://surgicalneurologyint.com/surgicalint-articles/13066/
Abstract
Background: Vagal paragangliomas (VPs) are rare tumors in the upper cervical region. Although surgical resection is the standard treatment for these tumors, it carries significant risks due to the tumor’s high vascularity and proximity to vital structures. Stereotactic radiosurgery (SRS) for skull base paraganglioma could be a minimally invasive alternative.
Case Description: We report the case of a 47-year-old man with a large, asymptomatic VP who was successfully treated with SRS with Gamma Knife Icon, which was performed in the parapharyngeal space (volume: 25.7 mL) using a marginal dose of 14 Gy to the 45% isodose line. This case illustrates the successful treatment of a lesion near the conventional limits (lower limit of C2 vertebral body) using noninvasive mask fixation. Excellent tumor control without neurological deficits was achieved for 25 months after SRS. The tumor volume decreased by 70% (final volume: 7.6 mL).
Conclusion: This study demonstrates the utility of Gamma Knife Icon, which facilitates optimal SRS for upper cervical lesions, including VPs.
Keywords: Gamma knife icon, Noninvasive treatment, Stereotactic radiosurgery, Vagal paraganglioma, Vagus nerve
INTRODUCTION
Paragangliomas, also known as glomus tumors, are rare neuroendocrine tumors originating from the neural crest that can grow in various regions, including the head, neck, thorax, abdomen, and adrenal glands.[
CASE PRESENTATION
History, examination, and imaging
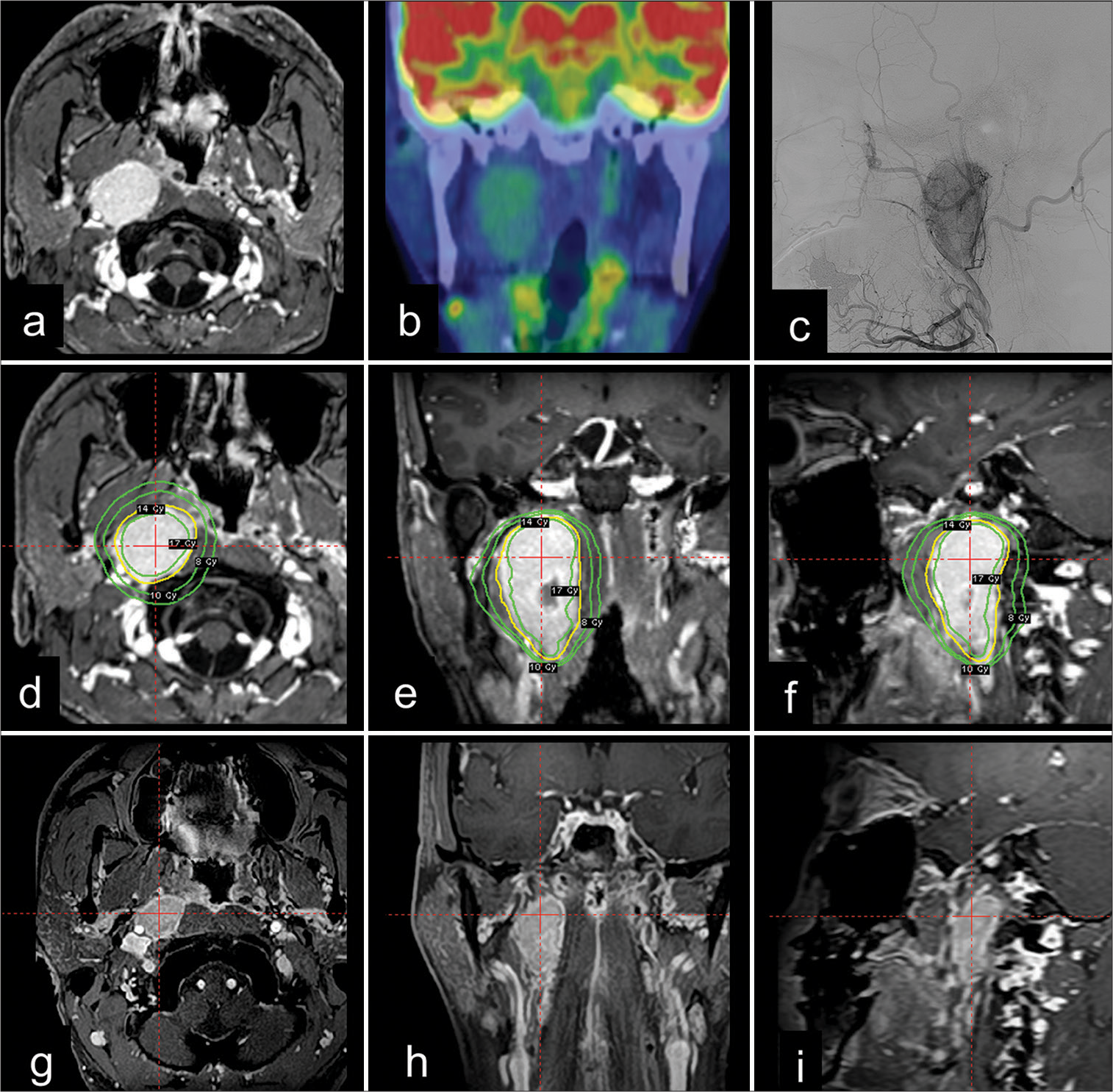
A 47-year-old male was referred to our hospital for investigation and treatment of a tumor-like lesion in the right parapharyngeal space, which was incidentally detected on computed tomography (CT) for head trauma. The patient did not present with any neurological deficits, and routine blood examinations, including catecholamine levels, yielded normal results. Gadolinium-enhanced magnetic resonance imaging (MRI) revealed a well-defined, enhancing, round mass localized between the pterygoid and longus capitis muscles (size, 35 × 29 × 55 mm; volume, 25.7 mL); [
Figure 1:
Vagal paraganglioma incidentally detected in a 47-year-old man. (a) Gadolinium-enhanced, T1-weighted magnetic resonance imaging (MRI) showing a well-enhanced tumor in the parapharyngeal space. (b) Positron emission tomography revealed increased fluorodeoxyglucose uptake within the lesion, with no evidence of metastasis to the other location. (c) Digital subtraction angiography showing the hypervascularity of the tumor, primarily supplied by the ascending pharyngeal artery. (d-f) Stereotactic radiosurgery (SRS) with Gamma Knife using mask fixation was performed, and radiosurgical planning images are shown in the axial, coronal, and sagittal planes. The yellow line indicates the 45% isodose line for the prescribed treatment dose of 14 Gy, and the green lines indicate the doses of 8, 10, and 17 Gy. (g-i) The patient’s clinical course was uneventful, and MRI revealed significant tumor shrinkage with a 70% decrease in volume 25 months after SRS.
Management
Considering the patient’s preference for nonsurgical treatment modalities, SRS using the Leksell Gamma Knife Icon was performed. Since the inferior margins of the tumor extended to the lower C2 vertebra, a thermoplastic mask (Icon Mask Nanor, Elekta AB) and cushion (Icon Patient Cushion Klarity, Elekta AB) were used for fixation. Leksell frame (Elekta AB) fixation was not applicable in this case due to potential shoulder collisions. Preoperatively, contrast-enhanced cone-beam CT images were obtained to confirm consistent patient positioning during irradiation, ensuring accurate tumor targeting. A single-fraction marginal dose of 14 Gy was then delivered to the 45% isodose line [
Outcome
The patient’s post-SRS course was uneventful. Serial MRIs revealed tumor shrinkage at 3 months and contrast defect of internal tumor necrosis at 6 months. At the latest follow-up (25 months after SRS), a significant tumor reduction was observed (size: 25 × 19 × 46 mm, volume: 7.6 mL, a decrease of 70%), [
DISCUSSION
Surgical resection of paragangliomas carries a potential risk of jeopardizing neurological function due to its high vascularity and the nature of being adjacent to critical structures. Consequently, the optimal treatment approach in such cases remains controversial. Radiation therapy offers an alternative or adjunctive therapy to surgical resection by hindering tumor progression and lowering surgical thresholds, which particularly benefits cases deemed difficult or unsuitable for surgery, cases of recurrent or unresectable tumors, or cases requiring palliative care.[
Recently, SRS has gained traction for its use against head-and-neck paragangliomas due to its ability to deliver high-dose, meticulously targeted radiation to tumor margins.[
Previously, head fixation for Gamma Knife-based SRS was limited to frame-based methods, restricting treatment for C1/C2 vertebrae.[
Despite the valuable insights offered in this study, certain limitations should be acknowledged. The absence of pathological confirmation and the relatively short follow-up period may have affected our findings. A further evaluation of larger patient cohorts is necessary to evaluate the efficacy of SRS for VPs and to explore the lower limits of SRS applicability for upper cervical lesions.
CONCLUSION
This report describes the first successful application of SRS in VP. SRS with mask fixation of the head and neck might be an alternative noninvasive treatment option for surgical resection, achieving favorable control of VP.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI; grant no. 22K20815 (to Motoyuki Umekawa).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ampil F, Sin A, Smith D, Richards T. GammaKnife radiosurgery for Fisch-classified jugulotympanic paragangliomas: Review of the measures and timing of treatment success. J Radiosurg SBRT. 2022. 8: 211-5
2. Casarim AL, Tincani AJ, Del Negro A, Aguiar CG, Fanni RV, Martins AS. Carotid body tumor: Retrospective analysis on 22 patients. Sao Paulo Med J. 2014. 132: 133-9
3. Gerosa M, Visca A, Rizzo P, Foroni R, Nicolato A, Bricolo A. Glomus jugulare tumors: The option of gamma knife radiosurgery. Neurosurgery. 2006. 59: 561-9
4. Gilbo P, Morris CG, Amdur RJ, Werning JW, Dziegielewski PT, Kirwan J. Radiotherapy for benign head and neck paragangliomas: A 45-year experience. Cancer. 2014. 120: 3738-43
5. Gottfried ON, Liu JK, Couldwell WT. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg Focus. 2004. 17: E4
6. Hellinger RL, Wolf A, Blach L, Kleinberg LR, Coy S. Long-term results of gamma knife radiosurgery for glomus tumors: An analysis of 32 patients. Cureus. 2021. 13: e18095
7. Hu K, Persky MS. Treatment of head and neck paragangliomas. Cancer Control. 2016. 23: 228-41
8. Jackson CG. Neurotologic skull base surgery for glomus tumors. Diagnosis for treatment planning and treatment options. Laryngoscope. 1993. 103: 17-22
9. Jackson CG, McGrew BM, Forest JA, Netterville JL, Hampf CF, Glasscock ME. Lateral skull base surgery for glomus tumors: Long-term control. Otol Neurotol. 2001. 22: 377-82
10. Künzel J, de Tristan J, Mantsopoulos K, Koch M, Baussmerth M, Zenk J. Experiences in the treatment of patients with multiple head and neck paragangliomas. Am J Otolaryngol. 2014. 35: 294-9
11. Lassen-Ramshad Y, Ozyar E, Alanyali S, Poortmans P, van Houtte P, Sohawon S. Paraganglioma of the head and neck region, treated with radiation therapy, a Rare Cancer Network study. Head Neck. 2019. 41: 1770-6
12. Lee SJ, Kim MS, Jeong YG, Lee SI, Jung YT, Sim JH. Gamma-knife radiosurgery of upper cervical spinal cord tumor. J Korean Neurosurg Soc. 2004. 36: 135-7
13. Maxwell AK, Mehta GU, Muelleman T, Barnard ZR, Hartwick T, Mak A. Hypofractionated robotic stereotactic radiosurgery for vagal paragangliomas: A novel treatment strategy for cranial nerve preservation. Otolaryngol Head Neck Surg. 2020. 162: 897-904
14. Newman H, Rowe JF, Phillips TL. Radiation therapy of the glomus jugulare tumor. Am J Roentgenol Radium Ther Nucl Med. 1973. 118: 663-9
15. Patel AK, Rodríguez-López JL, Hirsch BE, Burton SA, Flickinger JC, Clump DA. Long term outcomes with linear accelerator stereotactic radiosurgery for treatment of jugulotympanic paragangliomas. Head Neck. 2021. 43: 449-55
16. Suarez C, Rodrigo JP, Bödeker CC, Llorente JL, Silver CE, Jansen JC. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head Neck. 2013. 35: 1195-204
17. Tripathi M, Kumar N, Mukherjee KK. Pushing the limits of the Leksell stereotactic frame for spinal lesions up to C3: Fixation at the maxilla. Acta Neurochir (Wien). 2016. 158: 1691-5
18. van der Mey AG, Frijns JH, Cornelisse CJ, Brons EN, van Dulken H, Terpstra HL. Does intervention improve the natural course of glomus tumors? A series of 108 patients seen in a 32-year period. Ann Otol Rhinol Laryngol. 1992. 101: 635-42
19. Wang ML, Hussey DH, Doornbos JF, Vigliotti AP, Wen BC. Chemodectoma of the temporal bone: A comparison of surgical and radiotherapeutic results. Int J Radiat Oncol Biol Phys. 1988. 14: 643-8