- Department of Cerebrovascular Surgery, International Medical Center, Saitama Medical University, Hidaka, Japan
- Department of Pathology, International Medical Center, Saitama Medical University, Hidaka, Japan.
Correspondence Address:
Kaima Suzuki, Department of Cerebrovascular Surgery, International Medical Center, Saitama Medical University, Hidaka, Japan.
DOI:10.25259/SNI_11_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Atsushi Hashio1, Hiroki Sato1, Milan Lepić1, Kaima Suzuki1, Tsugumi Satoh2, Shin Nemoto1, Seiji Kuribara1, Yuhei Ito1, Shun Suzuki1, Ichi Lee1, Akio Teranishi1, Taro Yanagawa1, Toshiki Ikeda1, Hidetoshi Ooigawa1, Hiroki Kurita1. A technique for reconstruction of a giant extracranial internal carotid artery aneurysm: A technical note. 08-Mar-2024;15:80
How to cite this URL: Atsushi Hashio1, Hiroki Sato1, Milan Lepić1, Kaima Suzuki1, Tsugumi Satoh2, Shin Nemoto1, Seiji Kuribara1, Yuhei Ito1, Shun Suzuki1, Ichi Lee1, Akio Teranishi1, Taro Yanagawa1, Toshiki Ikeda1, Hidetoshi Ooigawa1, Hiroki Kurita1. A technique for reconstruction of a giant extracranial internal carotid artery aneurysm: A technical note. 08-Mar-2024;15:80. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12784
Abstract
Background: Surgery is effective for extracranial internal carotid artery (EICA) aneurysms. However, the risk of cranial nerve injury associated with surgical repair, such as graft-assisted resection and extracranial-intracranial bypass techniques, is relatively high. Here, we report two cases of surgical treatment for EICA aneurysms and describe the surgical techniques and strategies to avoid cranial nerve injury.
Methods: Two patients presented to our facility with an increasing cervical pulsatile mass and no neurological symptoms. Angiography showed a large aneurysm in the cervical internal carotid artery. Surgical treatment was performed to prevent rupture of the aneurysm. In both patients, the aneurysm was strongly attached to the vagus nerve. The aneurysm and vagus nerve were carefully dissected using a low-power bipolar (20 Malis; 3 watts), leaving connective tissue on the vagus nerve side.
Results: The aneurysm was detached from the vagus nerve without injury. Based on intraoperative findings, one patient underwent clipping, and the other underwent aneurysmectomy and primary closure for aneurysm obliteration and angioplasty. Both patients were discharged without any cranial nerve dysfunction.
Conclusion: The selection of a strategy based on intraoperative findings and low-power bipolar cutting is important for the treatment of extracranial carotid artery aneurysms to preserve cranial nerves.
Keywords: Aneurysmectomy, Bipolar cutting, Extracranial internal carotid artery aneurysm, Reconstruction, Vagus nerve
INTRODUCTION
Extracranial internal carotid artery (EICA) aneurysms are rare, with an incidence of <1% of all arterial aneurysms[
Conservative treatment has a high mortality rate (60–70%),[
Technical success of surgery is achieved in almost all cases; however, the risks of mortality and complications are significant.[
We report the two cases of patients with EICA who underwent aneurysmectomy and internal carotid artery (ICA) repair, emphasizing the technical aspects of improving safety and preserving the cranial nerves.
Written informed consent to publish this report was obtained from the patients before the submission process.
CASE DESCRIPTION
Case 1
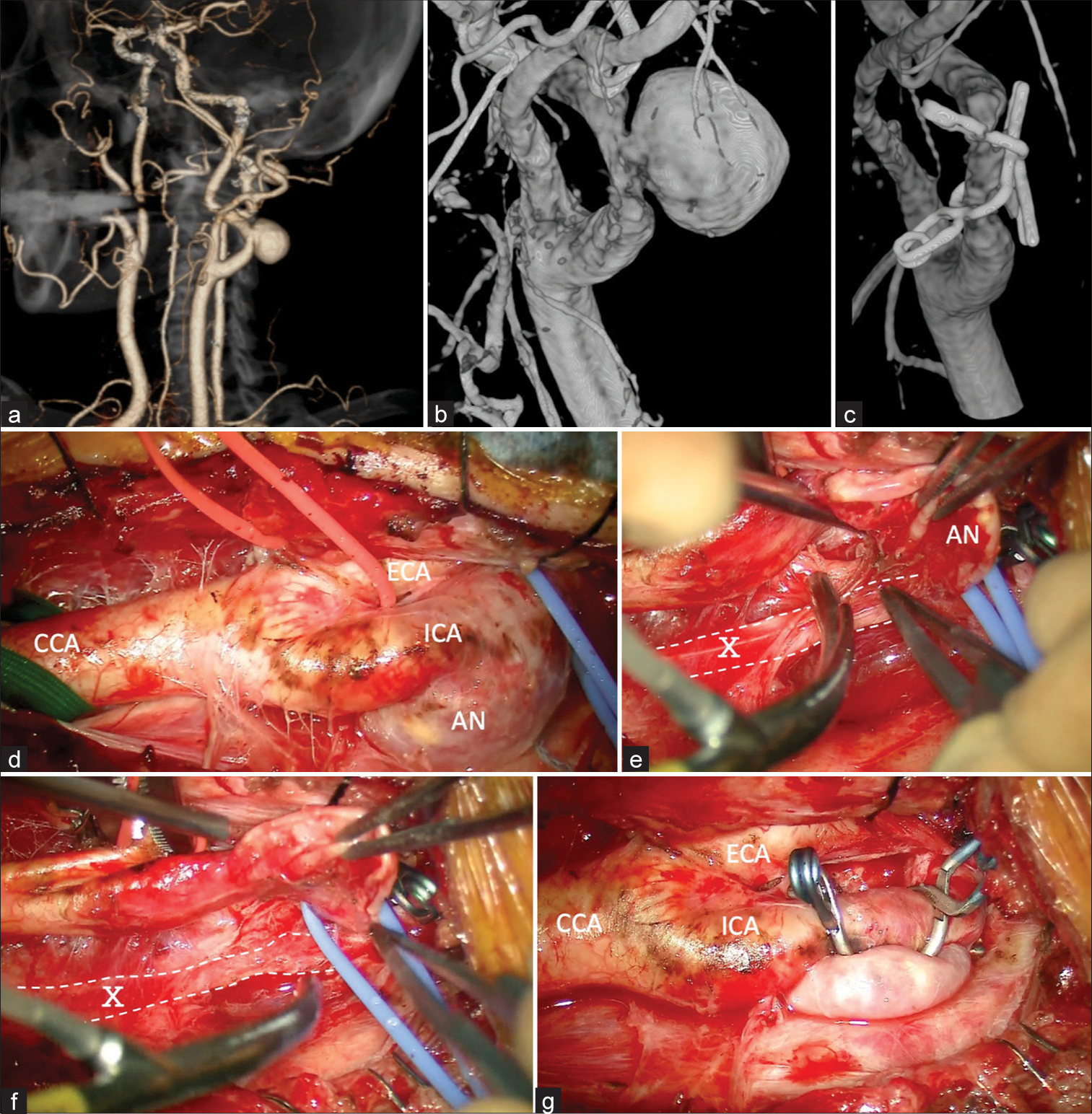
A 76-year-old woman presented to our institution with a pulsatile mass in her left neck. She had no neurological symptoms or history of cervical trauma, infection, surgery, or cerebral ischemic events. Three-dimensional computed tomography and digital subtraction angiography revealed an aneurysm measuring 18 mm in the left ICA of the neck [
Figure 1:
Case 1 (a) Three-dimensional computed tomography angiogram demonstrating a saccular aneurysm located at the left cervical ICA. (b and c) (b) Pre- and post-operative three-dimensional digital subtraction angiography showing the 18 mm aneurysm in the ICA (c) and disappearance of the aneurysm. (d-g) (d) Intraoperative photographs showing an exposed cervical ICA aneurysm, (e and f) before and after dissecting the aneurysm and vagus nerve using a cutting bipolar after clamping the CCA, ECA, ICA, and superior thyroid artery to prevent rupture of the aneurysm (g) two clips were used to clamp the aneurysm, and revascularization was performed. AN: Aneurysm, CCA: Common carotid artery, ECA: External carotid artery, ICA: Internal carotid artery, X: Vagus nerve.
Video 1
Case 2
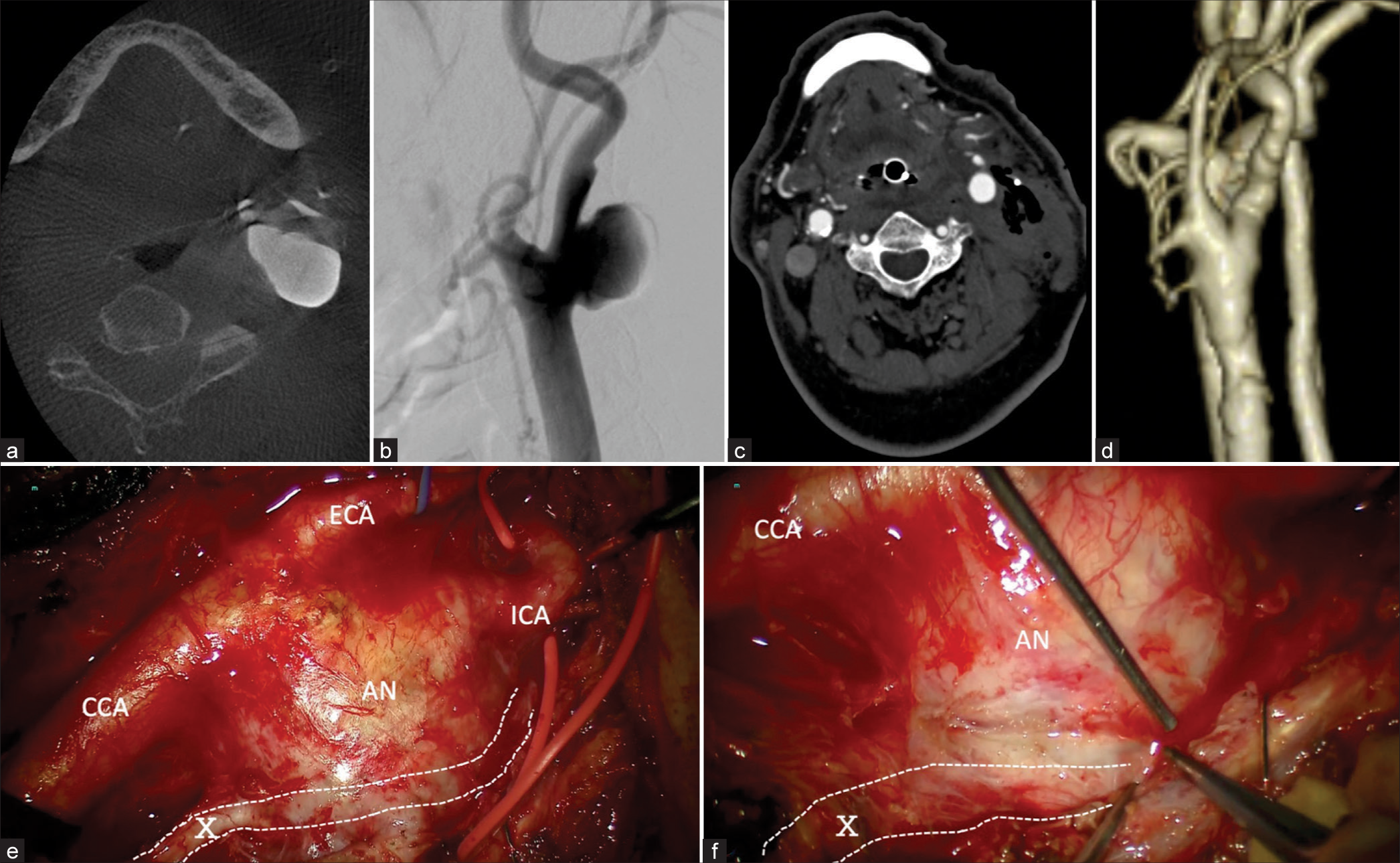
A 79-year-old male sought medical attention for a growing pulsatile mass in his left neck without any symptoms or pathological findings. Contrast-enhanced computed tomography showed a dilatation of the left cervical ICA with a partial luminal thrombus. Digital subtraction angiography revealed a giant aneurysm at the carotid bifurcation [
Figure 2:
Case 2 (a) Cone-beam computed tomography (CT) scan of the neck showing a mass on the left side of the neck, which appeared to be a partially thrombosed aneurysm (axial view). (b) During angiography, a saccular aneurysm measuring 2.1 cm is observed near the bifurcation site of the left common carotid artery into the ICA (lateral view). (c and d) Postoperative contrast-enhanced CT scans of the neck (c) and three-dimensional computed tomography angiogram (d) show the disappearance of the aneurysm. (e and f) Intraoperative photographs.(e) The connective tissue around the aneurysm is completely exfoliated . (f)The aneurysm was strongly adherent to the vagus nerve in some areas, although it was dissected using a cutting bipolar device to expose the aneurysm . AN: Aneurysm, CCA: Common carotid artery, ECA: External carotid artery, ICA: Internal carotid artery, X: Vagus nerve
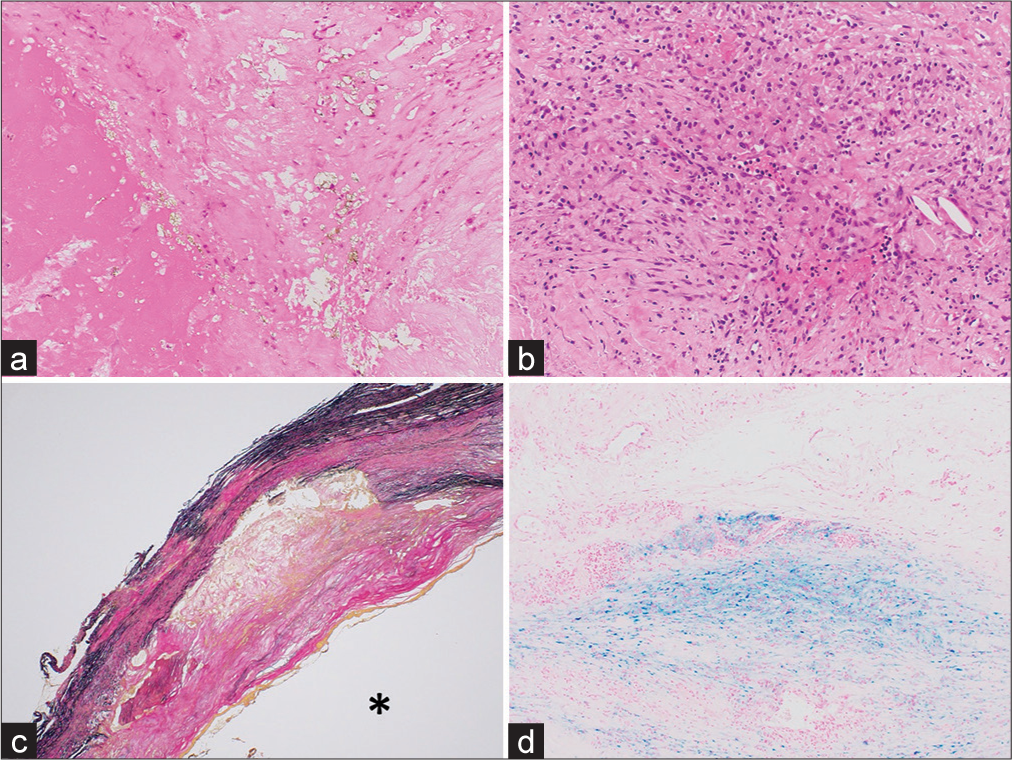
Figure 3:
Histopathological findings in Case 2. Hematoxylin and eosin staining (a: ×100, b: ×200) revealed calcification, cholesterin clefts, and granulation tissue-like parts of the aneurysm wall. Elastica van Gieson staining (c: ×2) showed a decrease in aneurysm wall elastic fibers and bleeding on the vessel wall (*: Vascular lumen). Berlin Blue staining (d: ×100) showed hemosiderin deposition in the vessel wall. The etiology of the aneurysm formation was considered to be arteriosclerosis.
DISCUSSION
We reported two surgical cases of EICA. The aneurysm and vagus nerve sometimes adhered to each other, and a low-power bipolar cut was useful to dissect the aneurysm without causing vagus nerve damage. In addition, for better treatment, flexible strategy changes based on intraoperative findings are essential.
Reportedly, 2.2–44% of the lower cranial nerve injuries are caused by surgical repair of the EICA.[
The natural history of the EICA remains to be elucidated. Ruptured aneurysms are thought to be very rare and potentially life-threatening. EICA commonly leads to transient ischemic attacks, cerebral infarction due to inter-arterial embolism, and pulsatile masses in the neck and pharynx. As the aneurysm enlarges, symptoms due to compression of surrounding biological structures, such as dysphagia and hoarseness, may occur.[
Several procedures for the treatment of EICA have been reported, such as direct suture of the defect after aneurysm resection, direct end-to-end anastomosis of the ICA or CCA after aneurysm resection, autologous or artificial vascular graft after aneurysm resection, or ligation of aneurysms under combined bypass.[
The most common cause of EICA is atherosclerosis, followed by arterial dissection and trauma.[
CONCLUSION
We reported an intraoperative technique to protect the vagus nerve in EICA surgery by dissecting the connective tissue between the aneurysm and the vagus nerve in the vicinity of the aneurysm and leaving the connective tissue around the vagus nerve. This technique may improve functional outcomes after EICA surgery. This case is also a reminder that proper selection of the procedure for aneurysm repair and reconstruction of the ICA and the device for dissection are important for this rare cervical vascular pathology.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000. 31: 702-12
2. Garg K, Rockman CB, Lee V, Maldonado TS, Jacobowitz GR, Adelman MA. Presentation and management of carotid artery aneurysms and pseudoaneurysms. J Vasc Surg. 2012. 55: 1618-22
3. Han Q, Zhou P, Huang Y. Surgical revascularization: Ligation of extracranial internal carotid artery and superficial temporal artery-to-middle cerebral artery bypass in patient with extracranial internal carotid aneurysm and hemorrhagic moyamoya disease. World Neurosurg. 2019. 126: 129-33
4. Hanada Y, Sasai H, Kamakura A, Nakamura M, Sakata Y, Miyahara H. Rupture of an internal carotid artery pseudoaneurysm after irradiation for a nasopharyngeal carcinoma--case report. Nihon Jibiinkoka Gakkai Kaiho. 2013. 116: 606-11
5. Hoffman ME, Squiers JJ, Hamandi M, Lanfear AT, Calligaro KD, Shutze WP. Systematic review of the influence of anatomy and aneurysm type on treatment choice and outcomes in extracranial carotid artery aneurysms. Ann Vasc Surg. 2022. 83: 349-57
6. Komine H, Teranishi A, Kayahara T, Take Y, Ikegami M, Kikkawa Y. Surgical management of a thromobotic extracranial carotid artery aneurysm: A case report. No Shinkei Geka. 2019. 47: 653-8
7. Kraemer CJ, Zhou W. Carotid aneurysm review. Int J Angiol. 2019. 28: 17-9
8. Maeda T, Sakai S, Osakabe M, Okawara M, Nomura T, Yamaguchi H. Giant saccular aneurysm of the cervical internal carotid artery treated with aneurysmectomy and side-to-end anastomosis. Surg Neurol Int. 2023. 14: 202
9. Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms. Stroke. 2000. 31: 896-900
10. Ohyama S, Takahashi S, Tamai K, Hori Y, Hirakawa Y, Hoshino M. Prevention of nerve root thermal injury caused by bipolar cauterization near the nerve roots. Spine. 2019. 44: E321-8
11. Okazaki T, Kanematsu Y, Shimada K, Korai M, Satomi J, Uno M. A single-center retrospective study with 5-and 10-year follow-up of carotid endarterectomy with patch graft. Neurol Med Chir. 2019. 59: 231-7
12. Pirvu A, Bouchet C, Garibotti FM, Haupert S, Sessa C. Mycotic aneurysm of the internal carotid artery. Ann Vasc Surg. 2013. 27: 826-30
13. Radak D, Davidović L, Vukobratov V, Ilijevski N, Kostić D, Maksimović Z. Carotid artery aneurysms: Serbian multicentric study. Ann Vasc Surg. 2007. 21: 23-9
14. Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2009. 2009: CD000160
15. Sharma RK, Asiri AM, Yamada Y, Kawase T, Kato Y. Extracranial internal carotid artery aneurysm-challenges in the management: A case report and review literature. Asian J Neurosurg. 2019. 14: 970-4
16. Takamizawa S, Morino M. Knowledge of anatomical relationships of the vagus nerve with internal jugular vein and common carotid artery in the neck necessary for vagus nerve stimulation VNS surgery. Jpn J Neurosurg. 2015. 24: 705-11
17. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018. 39: 213-60
18. Vitošević F, Milošević-Medenica S. Traumatic intracranial aneurysms associated with traffic accidents and endovascular management options. Serb J Neurosurg. 2022. 1: 27-32
19. Welleweerd JC, den Ruijter HM, Nelissen BG, Bots ML, Kappelle LJ, Rinkel GJ. Management of extracranial carotid artery aneurysm. Eur J Vasc Endovasc Surg. 2015. 50: 141-7
20. Welleweerd JC, Nelissen BG, Koole D, de Vries JP, Moll FL, Pasterkamp G. Histological analysis of extracranial carotid artery aneurysms. PLoS One. 2015. 10: e0117915
21. Yoneyama T, Kawashima A, Sugiura M, Yamaguchi K, Itou K, Namioka A. Technical options for the surgical management of extracranial carotid artery aneurysms. Three case reports. Neurol Med Chir. 2012. 52: 208-12
22. Zannetti S, Parente B, De Rango P, Giordano G, Serafini G, Rossetti M. Role of surgical techniques and operative findings in cranial and cervical nerve injuries during carotid endarterectomy. Eur J Vasc Endovasc Surg. 1998. 15: 528-31
23. Zhang Q, Duan ZQ, Xin SJ, Wang XW, Dong YT. Management of extracranial carotid artery aneurysms: 17 years’ experience. Eur J Vasc Endovasc Surg. 1999. 18: 162-5
24. Zwolak RM, Whitehouse WM, Knake JE, Bernfeld BD, Zelenock GB, Cronenwett JL. Atherosclerotic extracranial carotid artery aneurysms. J Vasc Surg. 1984. 1: 415-22