- Department of Neurological Surgery, Juntendo University Urayasu Hospital, Urayasu, Japan
- Department of Pathology, Juntendo University Urayasu Hospital, Urayasu, Japan
Correspondence Address:
Satoshi Tsutsumi, Department of Neurological Surgery, Juntendo University Urayasu Hospital, Urayasu, Japan.
DOI:10.25259/SNI_456_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hana Asagiri1, Satoshi Tsutsumi1, Hiroshi Izumi2, Kasumi Inami1, Motoki Yamataka1, Natsuki Sugiyama1, Hideaki Ueno1, Hisato Ishii1. A unique case of Sylvian arachnoid cyst complicated by chronic subdural hematoma. 01-Nov-2024;15:399
How to cite this URL: Hana Asagiri1, Satoshi Tsutsumi1, Hiroshi Izumi2, Kasumi Inami1, Motoki Yamataka1, Natsuki Sugiyama1, Hideaki Ueno1, Hisato Ishii1. A unique case of Sylvian arachnoid cyst complicated by chronic subdural hematoma. 01-Nov-2024;15:399. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13193
Abstract
Background: Arachnoid cysts (ACs) complicated by chronic subdural hematoma (CSDH) are a rare but distinct entity.
Case Description: A 27-year-old man previously diagnosed with Sylvian AC presented to the hospital with a persistent headache. He was not aware of any preceding head trauma. However, he frequently performed bench presses at the gymnasium, especially 4 weeks prior. The patient did not exhibit any neurological deficits at presentation. Computed tomography revealed slightly low-density areas in the right cerebral convexity. Magnetic resonance imaging revealed compressive masses in the right middle fossa and cerebral convexity. The patient underwent a craniotomy. Reflection of the dura mater exposed thickened arachnoid membrane. Making an incision resulted in the egress of fluid hematoma. The membrane separating the subdural hematoma and inner AC possessed fine vasculature and adjacent holes. Furthermore, there were fragile clots adhered to the inner wall of the cyst. Microscopic findings of the separating membrane were consistent with inflammatory granulation tissue, similar to those of the outer membrane of CSDH.
Conclusion: Exertional hypertension associated with the bench press may result in the rupture of fine arteries distributed over the AC wall. Under certain circumstances, the AC wall may transform into the outer membrane of CSDH.
Keywords: Arachnoid cyst, Chronic subdural hematoma, Exertional hypertension, Rupture of arachnoid cyst
INTRODUCTION
The human brain contains two types of arachnoid membranes: the outer and inner membranes. The former surrounds the whole brain, whereas the latter divides the subarachnoid spaces into nine cisterns.[
Microscopically, CSDH can be classified into four types, with inflammatory and hemorrhagic inflammation types being the most frequent.[
An exertional hypertension caused by acute high-intensity resistance exercises, such as the bench press, is reported to increase the intravascular pressure of the cerebral arteries.[
Herein, we report a case of Sylvian AC complicated by CSDH presenting with peculiar intraoperative and microscopic findings. In this patient, exertional hypertension caused by bench press was assumed to be the underlying cause.
CASE PRESENTATION
A 27-year-old man presented to the hospital with a 3-week history of headaches. The patient was an office worker who was unaware of any preceding head trauma. He had previously been diagnosed with an asymptomatic Sylvian AC at the age of 10 years when he suffered a simple head injury and underwent cranial computed tomography (CT) at a local hospital. Since then, the AC remained asymptomatic. He did not play any contact sport but frequently performed bench presses at the gymnasium, especially 4 weeks prior. At presentation, he was well-oriented and did not exhibit any neurological deficits. Cranial CT revealed slightly low-density areas in the right temporal, frontal, and parietal convexities, with the cerebellopontine angle and ambient cisterns dilated on the right side. In addition, smooth-contoured erosive changes were observed in the right temporal skull [
Figure 1:
Axial computed tomography scans at presentation, taken (a) at the level of the inferior horn of the lateral ventricle, (b) third ventricle, and (c) upper lateral ventricle, showing slightly low-density areas in the right temporal, frontal, and parietal convexities (a and c, arrows). The cerebellopontine angle and ambient cisterns show dilation on the right side (a and b, dashed arrow). (d) A bone-target image at the same level as Figure (a) shows smooth-contoured erosive changes in the right temporal skull (arrowheads).
Figure 2:
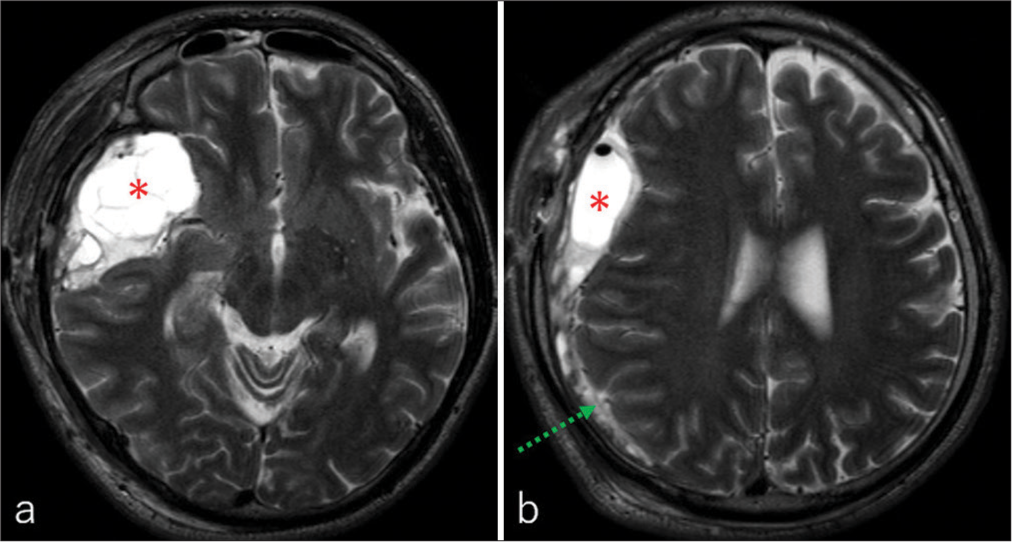
(a-c) Axial T1 and (d-f) T2-weighted magnetic resonance imaging taken at the level of the (a, d) inferior horn of the lateral ventricle, (b, e) third ventricle, and (c, f) upper lateral ventricle showing compressive masses in the right middle fossa (a-f, asterisk) and cerebral convexity (a-f, dashed arrow). The former lesion appears more hypointense, compared to the latter, on both T1-and T2-weighted sequences. Of note, linear structures with varying thicknesses and presenting hypointensity on both sequences can be observed in and around the former lesion (a-f, arrow).
Figure 4:
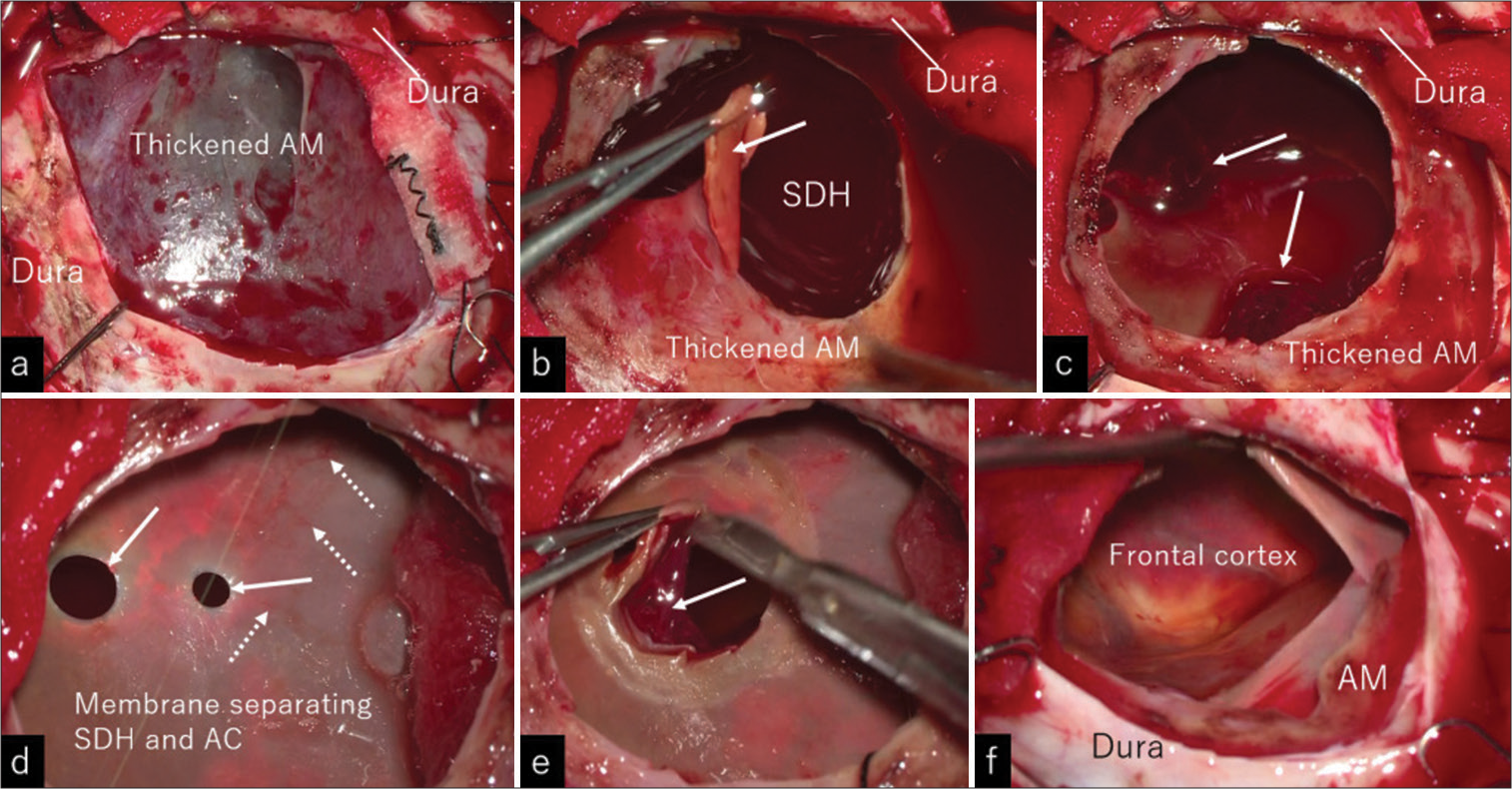
(a-f) Intraoperative photos showing the procedures in a step-by-step manner. (a) Reflection of the dura mater exposed unusually thickened arachnoid membrane. (b) Making an incision to the arachnoid resulted in the egress of dark red, fluid hematoma. The lower surface of the arachnoid was smooth (arrow). (c) After the evacuation of the fluid hematoma, soft and fragile clots were observed to scatter on the membrane separating the fluid hematoma and inner arachnoid cyst (arrows). (d) The separating membrane possessed fine vessels (dashed arrows) and two adjacent smooth-contoured, round holes (arrows). (e) Resection of the membrane exposed fragile clots loosely adhered to the entire inner cyst wall (arrow). (f) Circumferential removal of the clots revealed the intact frontal cortex. AC: Arachnoid cyst; AM: Arachnoid membrane; and SDH: Subdural hematoma.
Figure 5:
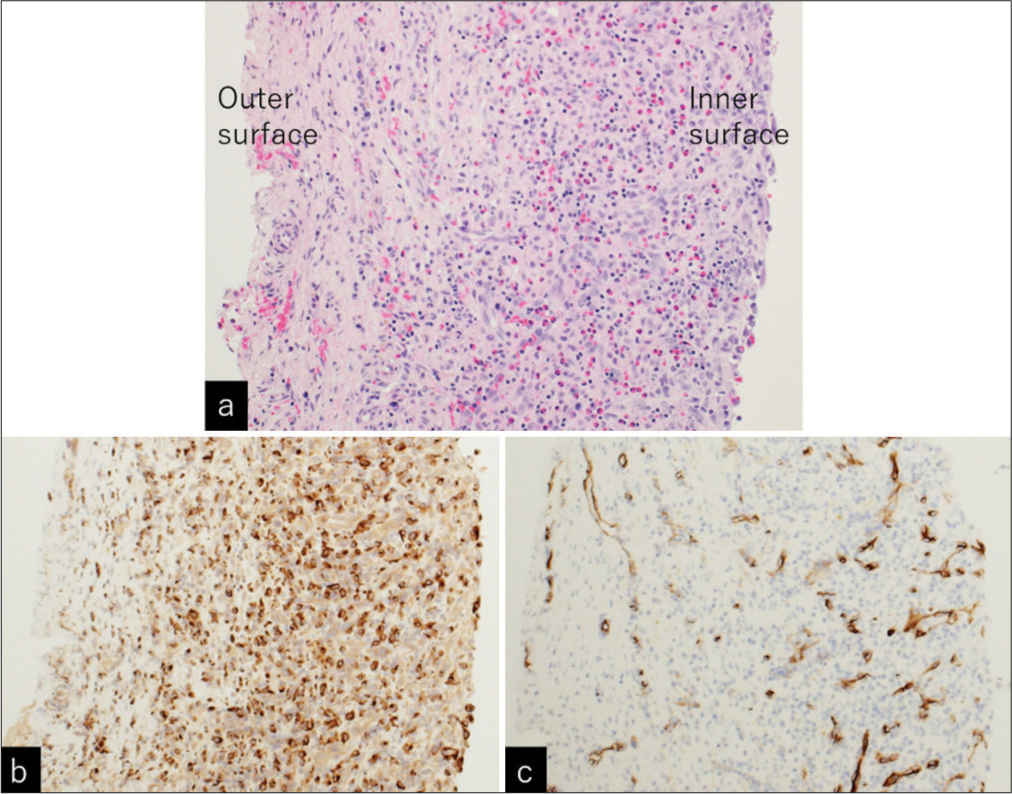
(a) Photomicrographs of the resected separating membrane showing lymphocytic and eosinophilic infiltrations, predominantly in the inner part of the membrane. Immunohistochemical examination showed positive staining for (b) CD68 ×20) and (c) CD34 (×20) predominantly in the inner part of the membrane, suggesting monocytic and microvascular proliferation. (a) Hematoxylin and eosin staining, ×20.
Figure 6:
Axial T2-weighted, magnetic resonance imaging, performed on postoperative day 5, at the level of the (a) third ventricle and (b) upper lateral ventricle showing less compressive, extra-axial cyst in the right Sylvian fissure (asterisk), and satisfactory evacuation of chronic subdural hematoma (dashed arrow).
DISCUSSION
The present patient was previously diagnosed with Sylvian AC and had been asymptomatic for a long period prior to the development of the headache. Neuroimaging examinations at the presentation, as well as subsequent intraoperative findings, indicated a fluidy subdural hematoma and adjacent cyst accompanied by hemorrhagic changes. Moreover, postoperative MRI revealed an extra-axial cyst within the Sylvian fissure. In addition, intraoperative and microscopic findings suggested the development of CSDH. Therefore, we hypothesized that the pre-existing Sylvian AC may have ruptured, followed by the formation of the CSDH. Given that the inflammatory changes, histologically similar to those of CSDH,[
The present patient was unaware of the preceding head trauma and had frequently performed bench presses 4 weeks before the presentation. Prior studies have indicated that exertional hypertension associated with the bench press can increase the intravascular pressure in the cerebral arteries.[
The diameter of the middle meningeal arteries has been reported to increase in patients with mature CSDHs.[
CONCLUSION
Exertional hypertension associated with the bench press may result in the rupture of fine arteries distributed over the AC wall. Under certain circumstances, the AC wall may transform into the outer membrane of CSDH.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Domenicucci M, Russo N, Giugni E, Pierallini A. Relationship between supratentorial arachnoid cyst and chronic subdural hematoma: Neuroradiological evidence and surgical treatment. J Neurosurg. 2009. 110: 1250-5
2. Gandhoke GS, Kaif M, Choi L, Williamson RW, Nakaji P. Histopathological features of the outer membrane of chronic subdural hematoma and correlation with clinical and radiological features. J Clin Neurosci. 2013. 20: 1398-401
3. Gregori F, Colista D, Mancarella C, Chiarella V, Marotta N, Domenicucci M. Arachnoid cyst in young soccer players complicated by chronic subdural hematoma: Personal experience and review of the literature. Acta Neurol Belg. 2020. 120: 235-46
4. Inoue K, Seker A, Osawa S, Alencastro LF, Matsushima T, Rhoton AL. Microsurgical and endoscopic anatomy of the supratentorial arachnoidal membranes and cisterns. Neurosurgery. 2009. 65: 644-65
5. Jensen TS, Olsen MH, Christoffersen C, Binderup T, Fugleholm K. The cellular composition of chronic subdural hematoma. Acta Neurochir (Wien). 2024. 166: 208
6. Lefferts WK, Hughes WE, Hefferman KS. Effect of acute high-intensity resistance exercise on optic nerve sheath diameter and ophthalmic artery blood flow pulsatility. J Hum Hypertens. 2015. 29: 744-8
7. Massimi L, Bianchi F, Benato A, Frassanito P, Tamburrini G. Ruptured Sylvian arachnoid cysts: An update on a real problem. Childs Nerv Syst. 2023. 39: 93-119
8. Poirrier AL, Ngosso-Tetanye I, Mouchamps M, Misson JP. Spontaneous arachnoid cyst rupture in a previously asymptomatic child: A case report. Eur J Paediatr Neurol. 2004. 8: 247-51
9. Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cyst. J Neuropathol Neurol. 1981. 40: 61-83
10. Takizawa K, Sorimachi T, Honda Y, Ishizaka H, Baba T, Osada T. Chronic subdural hematomas associated with arachnoid cysts: Significance in young patients with chronic subdural hematomas. Neurol Med Chir (Tokyo). 2015. 55: 727-34
11. Takizawa K, Sorimachi T, Ishizuka H, Osada T, Srivatanakul K, Momose H. Enlargement of the middle meningeal artery on MR angiography in chronic subdural hematoma. J Neurosurg. 2016. 124: 1679-83
12. Wu X, Li G, Zhao J, Zhu X, Zhang Y, Hou K. Arachnoid cyst-associated chronic subdural hematoma: Report of 14 cases and a systematic literature review. World Neurosurg. 2018. 109: e118-30
13. Zeng T, Shi SS, Lin TF. Chronic subdural hematoma associated with Sylvian arachnoid cyst in juvenile athletes: Report of two cases and literature review. Chin J Traumatol. 2011. 14: 174-7