- Department of Medicine, Byramjee Jeejeebhoy Medical College and Civil Hospital, Ahmedabad, Gujarat, India
- Department of Medicine, Government Hospital Palsana, Palsana, Gujarat, India
- Department of Neurosurgery, Byramjee Jeejeebhoy Medical College and Civil Hospital, Ahmedabad, Gujarat, India.
Correspondence Address:
Hariom Vaja, Department of Medicine, Byramjee Jeejeebhoy Medical College and Civil Hospital, Ahmedabad, Gujarat, India.
DOI:10.25259/SNI_424_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hariom Vaja1, Shubham Nayankumar Patel1, Abhishek Vadher2, Masum Patel1, Megh Bhaveshkumar Patel1, Jaimin Shah3. A unique presentation of Crouzon-like syndrome: Complex craniosynostosis in the absence of genetic mutations or familial predisposition – A case report. 08-Dec-2023;14:422
How to cite this URL: Hariom Vaja1, Shubham Nayankumar Patel1, Abhishek Vadher2, Masum Patel1, Megh Bhaveshkumar Patel1, Jaimin Shah3. A unique presentation of Crouzon-like syndrome: Complex craniosynostosis in the absence of genetic mutations or familial predisposition – A case report. 08-Dec-2023;14:422. Available from: https://surgicalneurologyint.com/surgicalint-articles/12658/
Abstract
Background: Crouzon syndrome is a rare genetic disorder characterized by premature fusion of skull sutures during skull development, resulting in various craniofacial abnormalities and complex craniosynostosis is a condition in which more than one such sutures of the skull fuse prematurely.
Case Description: Herein, we present a case of a 5-year-old male diagnosed with Crouzon-like syndrome and complex craniosynostosis involving multiple cranial sutures, including metopic, sagittal, coronal (right and left), and lambdoid sutures, and without any identifiable mutations on karyotyping. The patient underwent successful surgical intervention with a satisfactory outcome, highlighting the importance of early diagnosis and intervention to prevent or minimize associated neurological manifestations and craniofacial abnormalities.
Conclusion: Our case report underscores the involvement of multiple cranial sutures in complex craniosynostosis and the absence of identifiable mutations or family history of similar craniofacial abnormalities, providing important insights into the diagnosis and management of this condition.
Keywords: Complex craniosynostosis, Cranial reconstruction, Crouzon syndrome, Genetic disorders, Neurology, Pediatric neurology
INTRODUCTION
Craniosynostosis refers to the condition where one or more sutures close prematurely. Premature closure of sutures can restrict or impede the growth of the brain within the affected area of the skull, while growth continues in areas where sutures remain open. If multiple sutures close prematurely, it can result in an atypical skull shape, even though the brain reaches its typical size. However, when restricted suture closure limits the brain’s space to grow, it can cause increased pressure within the skull, leading to various neurological manifestations.[
Complex craniosynostosis can give rise to a range of symptoms and complications, including but not limited to severe visual acuity impairment, speech and cognitive developmental delay, stunted height growth, and low weight gain. Other symptoms may include hypertelorism, proptosis, bilateral optic nerve compression, and irritability.[
Untreated craniosynostosis can result in facial abnormalities, sensory, respiratory, and neurological impairments, ocular anomalies, and psychological disruptions. Effective management of craniosynostosis requires early detection, skilled surgical interventions, postoperative care, and appropriate follow-up.[
In our case, the patient presented with a fusion of all the five sutures usually involved in complex craniosynostosis, that is, metopic, bi-coronal, sagittal, and lambdoid, leading to severe symptoms and complications; additionally, the patient has a normal male karyotype and showed no known mutations in polymerase chain reaction (PCR) or craniofacial deformities in the family history. In the subsequent section, we will discuss the case in detail.
CASE PRESENTATION
A 5-year-old male child was admitted to the hospital with notable craniofacial abnormalities, including a decreased anteroposterior length of the skull, prominent bulging of the eye, and congenital blindness. The child’s medical history revealed a neonatal jaundice episode shortly after birth, requiring an 8-day incubation period. Despite being born with a weight of 3.4 kg, the child exhibited failure to thrive and bilateral vision loss since birth. Additional physical examination findings included low-set ears and laryngomalacia.
At three months of age, the patient experienced a severe episode of bronchopneumonia, which was successfully treated with tazobactam+ceftriaxone, amikacin, and amoxicillin+clavulonic acid. At the age of 2.5 years, the patient had a history of febrile seizures preceded by an aura characterized by highly irritable and inconsolable crying, along with having a mild craniofacial deformity. It was at this age that the patient was suspected to have craniosynostosis, as suggested at a local hospital in their hometown. However, the patient first presented to our hospital at the age of 5 years with the complaint of skull deformity, reduced vision, and failure to achieve cognitive milestones, and it was then that surgical intervention was performed on the patient.
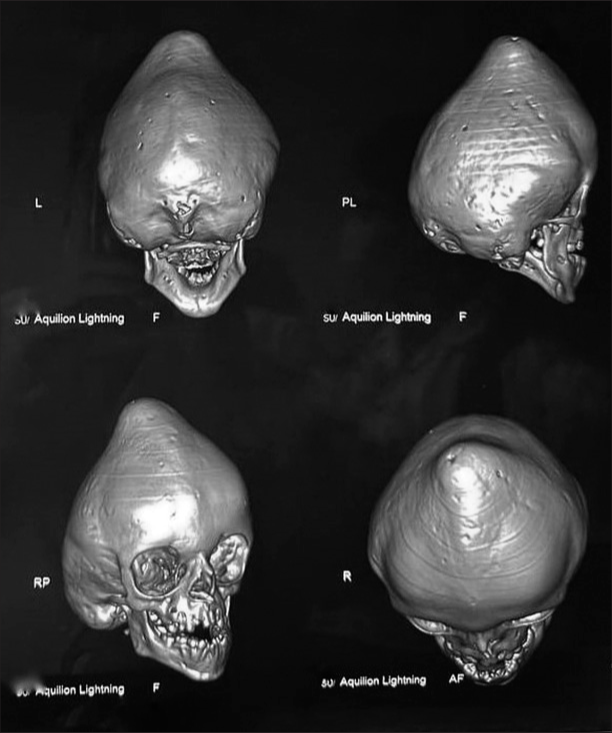
In addition to cognitive impairment and irritability, the patient exhibited significantly reduced visual acuity and proptosis in both eyes. The physical examination also revealed abnormal dentition, narrow orbits, maxillary hypoplasia, brachycephaly, plagiocephaly, trigonocephaly, turricephaly, and hypertelorism. These findings were attributed to premature fusion of the sagittal, metopic, bicoronal, and lambdoid sutures, as confirmed by 3D computed tomography (CT) reconstruction [
Figure 1:
Preoperative three-dimensional computed tomography (3D-CT) reconstruction of head. This figure displays a 3D-CT reconstruction of the patient’s skull bones before surgical intervention. The image reveals several craniofacial deformities, including brachycephaly, plagiocephaly, trigonocephaly, turricephaly, abnormal dentition, and hypertelorism, which are attributed to the fusion of the sagittal, metopic, bi-coronal, and lambdoid sutures.
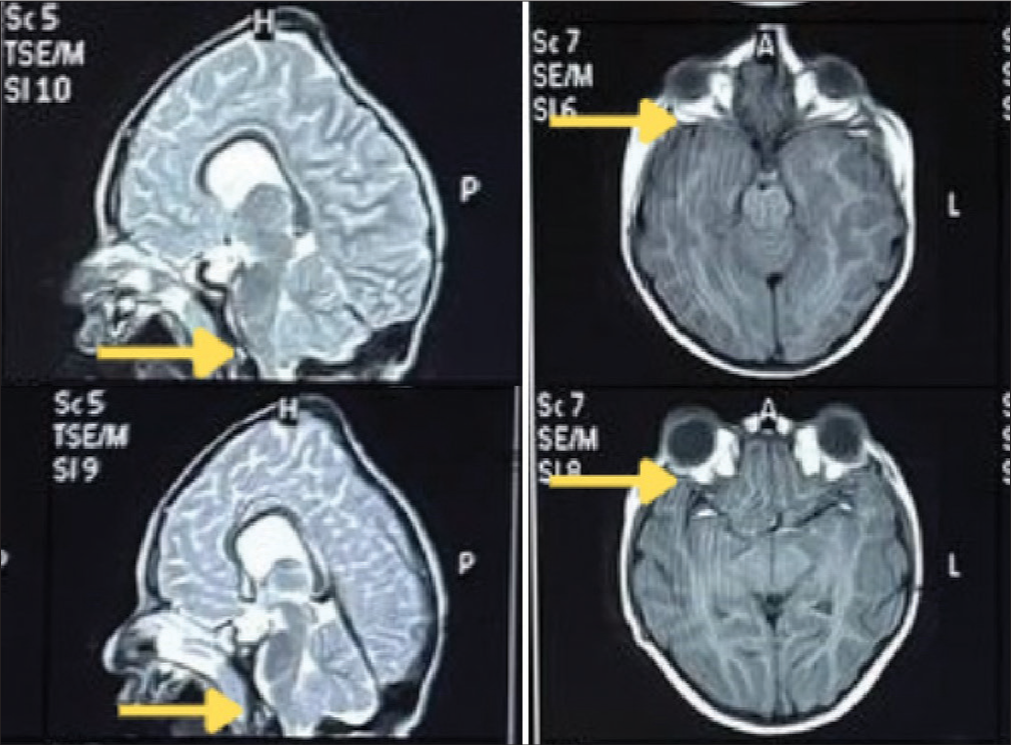
Figure 2:
Magnetic resonance imaging (MRI) scans showing optic nerve compression and tonsillar herniation. This figure shows the MRI scans showing intracranial optic nerve compression and tonsillar herniation. The left column displays T2-weighted MRI images showing tonsillar herniation (marked by yellow arrows). The T1-weighted MRI images in the right column indicate shallow orbits and optic nerve compression (marked by yellow arrows).
Based on the involvement of multiple sutures in craniosynostosis, as well as the presence of shallow orbits, hypertelorism, proptosis, abnormal dentition, and midface retrusion, a diagnosis of “Crouzon-like syndrome with Complex Craniosynostosis” was made.
The patient underwent a surgical procedure that involved a bi-coronal skin incision, pericranial graft harvesting, bicoronal bony flap raise, excision of the turricephalic bone, and pericranial duroplasty with rearrangement of bony fragments. During the postoperative period, the patient experienced a convulsive episode characterized by clonic movement in the left hand, twitching in the left angle of the mouth, and, subsequently, the left leg. Levetiracetam was initiated for the management of suspected focal seizures.
DISCUSSION
Background
Craniosynostosis, characterized by the premature fusion of cranial sutures, exhibits varying incidence rates depending on the type of suture involved. Sagittal, coronal, metopic, and lambdoid sutures have approximate incidence rates of 60%, 25%, 15%, and 5%, respectively.[
Observations
Our patient presented with brachycephaly, turricephaly, midface retrusion, hypertelorism, shallow orbits, and bilateral intracanalicular optic nerve compression, leading to reduced cognitive ability and severely impaired vision. Surgical intervention was performed, involving a bi-coronal skin incision, pericranial graft harvesting, bi-coronal bony flap raise, excision of the turricephalic bone, and pericranial duroplasty with rearrangement of bony fragments. Levetiracetam was administered postoperatively to control suspected focal seizures.
The primary challenge encountered in this case pertained to the delayed presentation of the patient to our medical facility. At the time of initial evaluation, the patient exhibited advanced symptomatic craniosynostosis, necessitating surgical intervention. The surgical objective was to mitigate further impairment of the patient’s visual acuity and facilitate optimal cognitive development, as both had been compromised due to the craniosynostosis condition.
Another challenge in this case was the absence of a definitive cause. While autosomal dominant heredity accounts for the majority of craniosynostosis cases, approximately 25% are attributed to spontaneous mutations.[
Turricephaly, also known as oxycephaly, is associated with Arnold Chiari Type I malformation in approximately 75% of cases. However, no such malformation was detected in the patient under consideration.[
Clinical approach
In young patients presenting with cranial anomalies and exorbitism, CS should be considered as a crucial diagnosis. Maxillary hypoplasia in these patients can lead to functional difficulties such as increased intracranial pressure, corneal exposure, and obstructive sleep apnea.[
Detailed examination should include the following factors:
Cranial anomalies Cardiac anomalies Airway examination Exposure keratopathy Papilledema/optic atrophy.
Employing robust imaging techniques is imperative for accurately identifying and assessing the extent of anomalies to formulate an appropriate surgical and medical management plan.
Imaging
Three-dimensional ultrasonography during pregnancy can detect premature closure of skull sutures.[
Genetic testing
Genetic testing plays a significant role in diagnosing craniosynostosis, with a gain of function mutation observed in over half of the cases.[
Management
Optimal management of patients with CS requires a multidisciplinary team consisting of a craniofacial surgeon, neurosurgeon, oculofacial plastic surgeon, head and neck surgeon, oral and maxillofacial surgeon, and pediatrician. Prompt diagnosis and management are crucial in preventing complications, the absence of which was one of the challenges that we faced. CS can be differentiated from other craniosynostoses by the presence of normal extremities, normal intellect, parrot beak nose, maxillary hypoplasia, and exorbitism;[
The management of CS involves acute and surgical measures. Acute management includes treating raised intracranial pressure, ventriculoperitoneal shunt placement for hydrocephalus, tarsorrhaphy for exposure keratopathy, and tracheostomy for severe maxillary or mandibular hypoplasia.
Surgical management aims to correct craniofacial anomalies, such as cranial vault remodeling, orbital rim and midface advancement, and mandibular advancement. The timing and order of surgical interventions depend on the degree and severity of craniofacial abnormalities.
Surgical management
Surgical management plays a critical role in the treatment of CS and complex craniosynostosis. In the presented case study, cranial vault remodeling and correction of facial abnormalities were the main surgical procedures.[
The surgical treatment of craniosynostosis is essential to achieve the best functional and esthetic results for the patient. McCarthy et al.’s procedure is a commonly used standard strategy.[
Modifications to procedures should be based on individual patient’s functional and cosmetic requirements. A multidisciplinary team consisting of craniofacial surgeons, neurosurgeons, oculofacial plastic surgeons, head and neck surgeons, oral and maxillofacial surgeons, and pediatricians should be involved to ensure optimal patient care.[
While distraction osteogenesis is an alternative procedure for repairing craniosynostosis, external distraction device placement may cause patient discomfort and inconvenience.[
CONCLUSION
The patient’s condition was characterized by late presentation to the clinic, early cranial suture fusion, and aberrant craniofacial development. Clinical examination, imaging, PCR, and karyotyping were used to make the diagnosis. The craniosynostosis was successfully repaired surgically. The patient’s motor and linguistic abilities improved, as did the IQ and the overall quality of life with reduced irritability.
The report emphasizes the involvement of all five cranial sutures normally associated with complex craniosynostosis and the absence of genetic abnormalities or family history of similar craniofacial abnormalities.
Ethical approval
Not applicable
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Al-Namnam NM, Hariri F, Thong MK, Rahman ZA. Crouzon syndrome: Genetic and intervention review. J Oral Biol Craniofac Res. 2019. 9: 37-9
2. Bhattacharjee K, Rehman O, Venkatraman V, Kikkawa D, Bhattacharjee H, Gogoi R. Crouzon syndrome and the eye: An overview. Indian J Ophthalmol. 2022. 70: 2346-54
3. Blaser SI, Padfield N, Chitayat D, Forrest CR. Skull base development and craniosynostosis. Pediatr Radiol. 2015. 45: S485-96
4. Czerwinski M, Kolar JC, Fearon JA. Complex craniosynostosis. Plast Reconstr Surg. 2011. 128: 955-61
5. Dicus Brookes C, Golden BA, Turvey TA. Craniosynostosis syndromes. Atlas Oral Maxillofac Surg Clin North Am. 2014. 22: 103-10
6. Greenberg MS, editors. Handbook of neurosurgery. United States: Thieme Medical Publishers; 2020. p.
7. Greenwood J, Flodman P, Osann K, Boyadjiev SA, Kimonis V. Familial incidence and associated symptoms in a population of individuals with nonsyndromic craniosynostosis. Genet Med. 2014. 16: 302-10
8. Johnson D, Wilkie AO. Craniosynostosis. Eur J Hum Genet. 2011. 19: 369-76
9. Kajdic N, Spazzapan P, Velnar T. Craniosynostosis-recognition, clinical characteristics, and treatment. Bosn J Basic Med Sci. 2018. 18: 110-6
10. McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992. 89: 1-8
11. McCarthy JG, Warren SM, Bernstein J, Burnett W, Cunningham ML, Edmond JC. Parameters of care for craniosynostosis. Cleft Palate Craniofac J. 2012. 49:
12. Renier D, Cinalli G, Lajeunie E, Arnaud E, Marchac D. Oxycephaly, a severe craniosynostosis. Apropos of a series of 129 cases. Arch Pediatr. 1997. 4: 722-9
13. Rostamzad P, Arslan ZF, Mathijssen IM, Koudstaal MJ, Pleumeekers MM, Versnel SL. Prevalence of ocular anomalies in craniosynostosis: A systematic review and meta-analysis. J Clin Med. 2022. 11: 1060
14. Sawh-Martinez R, Steinbacher DM. Syndromic craniosynostosis. Clin Plast Surg. 2019. 46: 141-55
15. Shruthi NM, Gulati S. Craniosynostosis: A pediatric neurologist’s perspective. J Pediatr Neurosci. 2022. 17: S54-60
16. Yang J, Tao T, Liu H, Hu ZL. Inherited FGFR2 mutation in a Chinese patient with Crouzon syndrome and luxation of bulbus oculi provoked by trauma: A case report. BMC Ophthalmol. 2019. 19: 209