- Department of Neurosurgery, Hospital Zambrano Hellion, TecSalud, San Pedro Garza García, Nuevo León, Mexico
- Department of Neurosurgery, National Institute of Pediatrics, Mexico City, Mexico
- Department of Neuro-Radiology, Hospital Zambrano Hellion, TecSalud, San Pedro Garza García, Nuevo León, Mexico.
Correspondence Address:
J. Javier Cuellar-Hernández, Department of Neurosurgery, Hospital Zambrano Hellion, TecSalud, San Pedro Garza, Mexico.
DOI:10.25259/SNI_59_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: J. Javier Cuellar-Hernández1,2, Omar R. Ortega-Ruiz1, Ana Guadalupe Rodriguez-Armendariz1, Carlos Daniel Castillo-Acevedo1, Luis Alejandro Pérez-Ruano1, Enrique Caro-Osorio1, Azalea Garza-Baez3. Accurate preoperative diagnosis of a Rathke cleft cyst with the aid of a novel classification for sellar cystic lesions and a diagnostic algorithm decision: Tools for differentiating cystic sellar lesions with a representative case. 05-Apr-2024;15:120
How to cite this URL: J. Javier Cuellar-Hernández1,2, Omar R. Ortega-Ruiz1, Ana Guadalupe Rodriguez-Armendariz1, Carlos Daniel Castillo-Acevedo1, Luis Alejandro Pérez-Ruano1, Enrique Caro-Osorio1, Azalea Garza-Baez3. Accurate preoperative diagnosis of a Rathke cleft cyst with the aid of a novel classification for sellar cystic lesions and a diagnostic algorithm decision: Tools for differentiating cystic sellar lesions with a representative case. 05-Apr-2024;15:120. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12843
Abstract
Background: Rathke’s cleft cyst (RCC) is a benign lesion in the sellar and suprasellar compartments. Similarly, pituitary adenomas can present with cystic morphology, making it a differential diagnosis when evaluating a patient with a cystic lesion in the sellar region. Surgical goals differ between RCCs and pituitary adenomas as the first can achieve remission of symptoms with cyst decompression in contrast to pituitary adenomas where complete resection would be the main goal. Imaging analysis alone may not be sufficient to define a preoperative surgical plan. The combination of imaging and conjoined use of validated tools may provide valuable insights to the clinician when defining a surgical approach.
Case Description: We present a case of a 27-year-old male with a 3-month history of visual disturbances and headaches. Magnetic resonance imaging showed a cystic lesion in the sellar compartment with compression of nearby structures. The authors were able to accurately diagnose this sellar lesion as an RCC with the conjoined aid of two classifications proposed in the literature. Cyst evacuation was performed with relief of symptoms and improved visual outcomes at follow-up.
Conclusion: While cystic adenomas can require total resection for cure, RCCs can show marked improvement with partial resection and evacuation of its contents. An accurate preoperative diagnosis can lead the surgeon to opt for the best surgical approach.
Keywords: Classification, Cystic adenoma, Endoscopic, Magnetic resonance imaging, Rathke cleft cyst
INTRODUCTION
Rathke’s cleft cysts (RCCs), classified as benign sellar and suprasellar lesions, trace their origin to the epithelial remnants of Rathke’s pouch with a peak incidence within the age range of 30–50 years.[
RCCs can share radiologic and clinical characteristics with other sellar cystic lesions as cystic pituitary adenomas. However, as cyst evacuation may provide symptomatic relief in RCCs, this might not be sufficient in pituitary adenomas. Therefore, surgical goals must be tailored for each patient undergoing surgery for sellar cystic lesions.[
In this context, we present a case involving an RCC in a 28-year-old male, highlighting the importance of an accurate preoperative diagnosis based on radiological characteristics supported by the conjoined use of an innovative classification scheme for sellar cystic lesions and a diagnostic tree decision as an alternative to differentiate between cystic sellar lesions.
CASE PRESENTATION
A 27-year-old man presented with chronic severe headaches with a history of pharmacological treatment without improvement and a 3-month history of progressive deterioration in visual acuity with blurring of his left-sided vision. On neurological examination, visual acuity in the right eye was reported to be 20/60 and 20/80 in the left eye with 2 mm isochoric pupils, normal light reflex, and bitemporal hemianopia. Extraocular movements were of full range, and fundus examination was within normal limits.
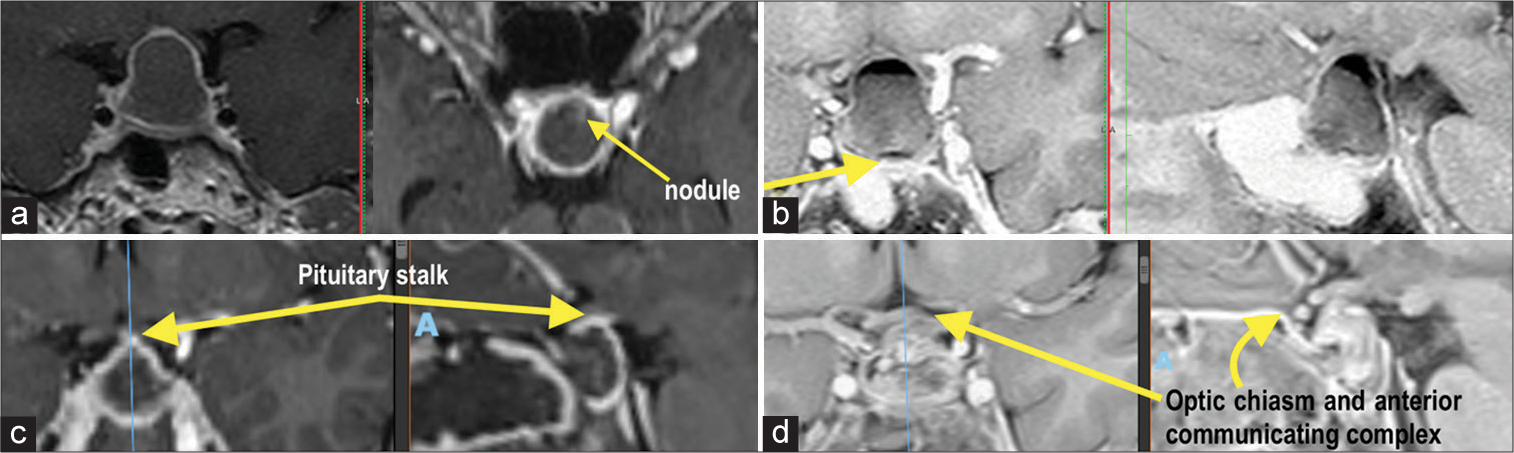
A pituitary hormonal assay was carried out, revealing panhypopituitarism. MRI was deemed necessary, showing an intrasellar cystic image with peripheral enhancement with an intracystic nodule without a septum or compartments. Displacement of the optic chiasm and the anterior communicating complex were observed, as well as intralesional fluid level [
Figure 1:
Pre and postoperative magnetic resonance imaging. (a) T1 gadolinium with an intrasellar cystic image with peripheral enhancement, intracystic nodule without septum or compartments. (b) Inverted T2 showing the displacement of the chiasm and the anterior communicating complex as well as the intra-lesional fluid level, arrow in figure b refers to “nodule” too. (c) Immediate postoperative T1 gadolinium with evidence of an intact pituitary stalk as well as persistence of peripheral enhancement that may correspond to the pituitary gland. (d) T2 inverted with improvement in the disposition of the chiasm and the anterior communicating complex.
Surgery
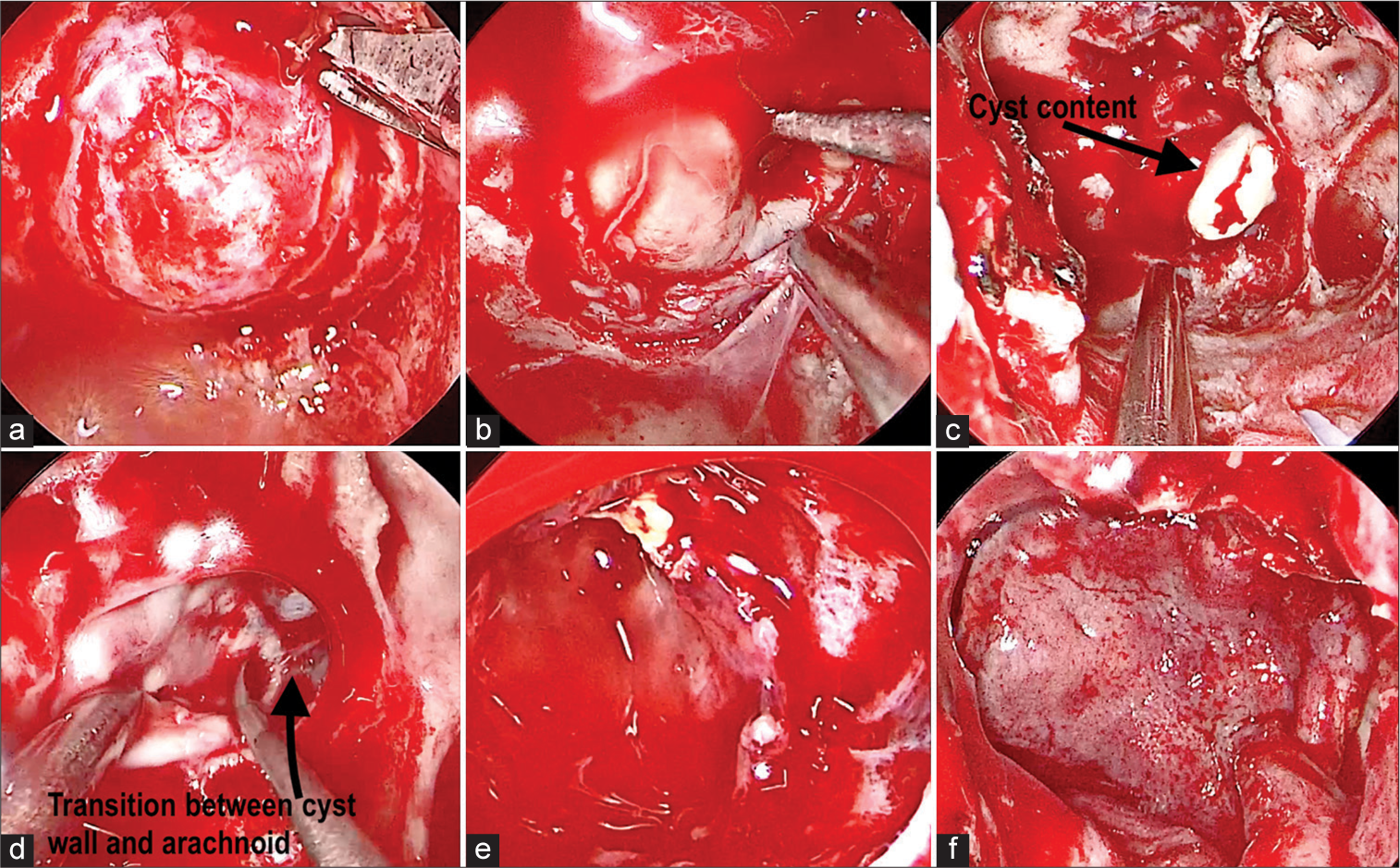
The patient underwent a transsphenoidal endoscopic endonasal approach, allowing the identification of a cystic intrasellar tumor with thickened walls and viscous xanthochromic content with displacement of the pituitary tissue without sellar diaphragm compromise. The tumor was fully resected without complications or incidents [
Figure 2:
Intraoperative images of the endoscopic endonasal approach. (a) U-shaped dural incision. (b) Dissection of the anterior, lateral, and inferior walls of Rathke’s cleft cyst capsule. (c) Drainage of cyst contents. (d) Dissection and total removal of the capsule. (e) Surgical bed demonstrating total resection of the capsule with integrity of the arachnoid membrane and residual pituitary. (f) Nasoseptal flap rotation.
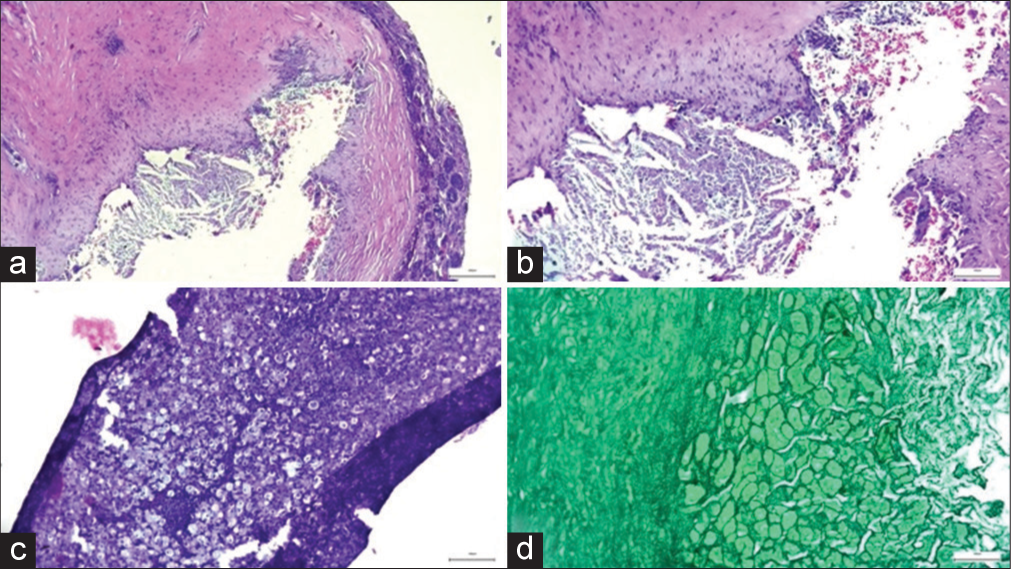
Figure 3:
(a-c) A predominantly lymphocytic inflammatory process is identified, with macrophages with clear and extensive cytoplasm (xanthocytes) and giant cells within a fibrous wall, which does not show epithelial lining, and displaces the normal pituitary gland. (d) Reticulin stain confirms the normal architectural distribution of the pituitary gland.
DISCUSSION
Before surgery and histopathological analysis, cystic epithelial lesions of sellar or parasellar location can be classified into RCCs, epithelial cysts, epidermoid cysts, dermoid cysts, and craniopharyngiomas.[
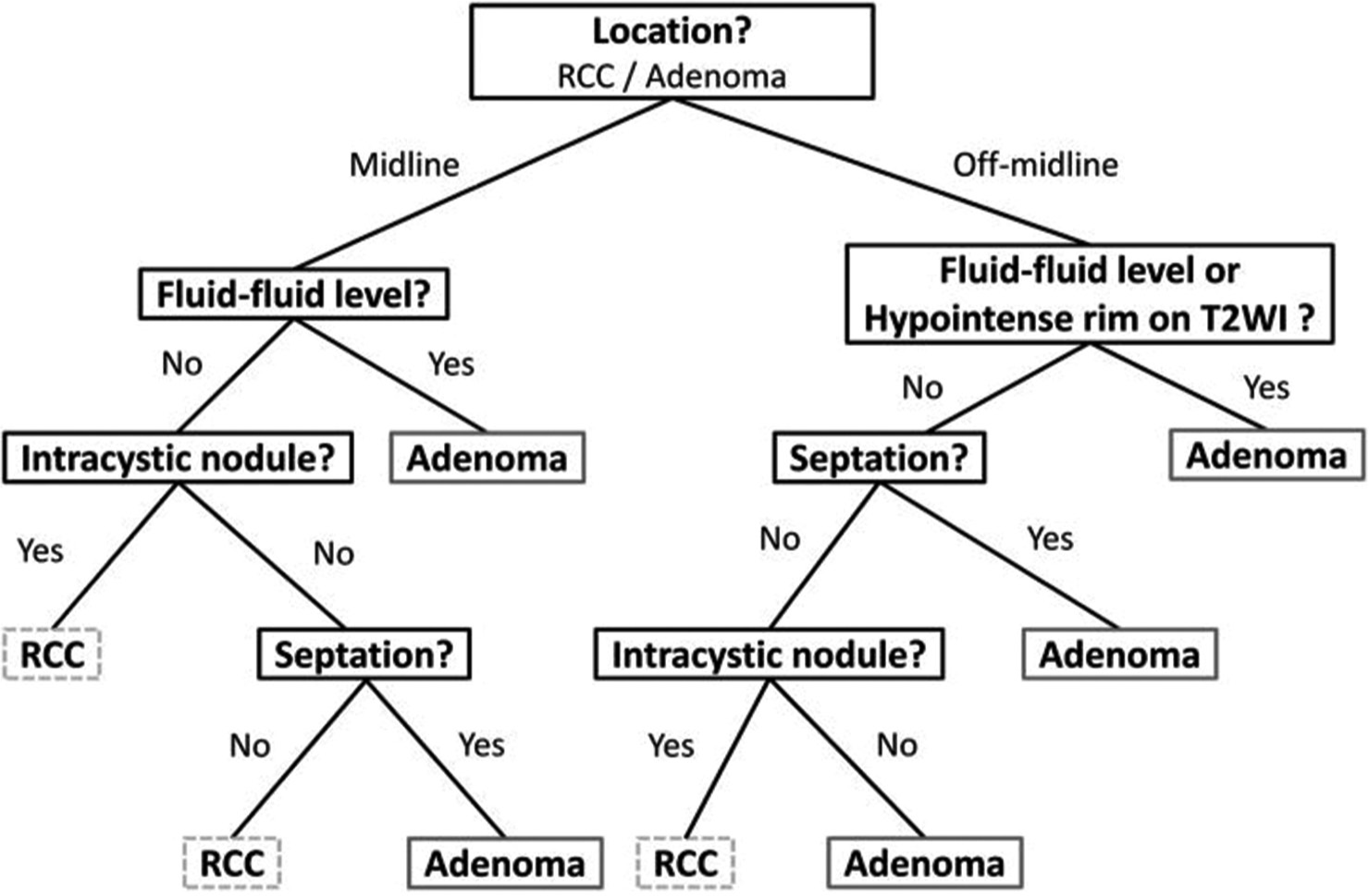
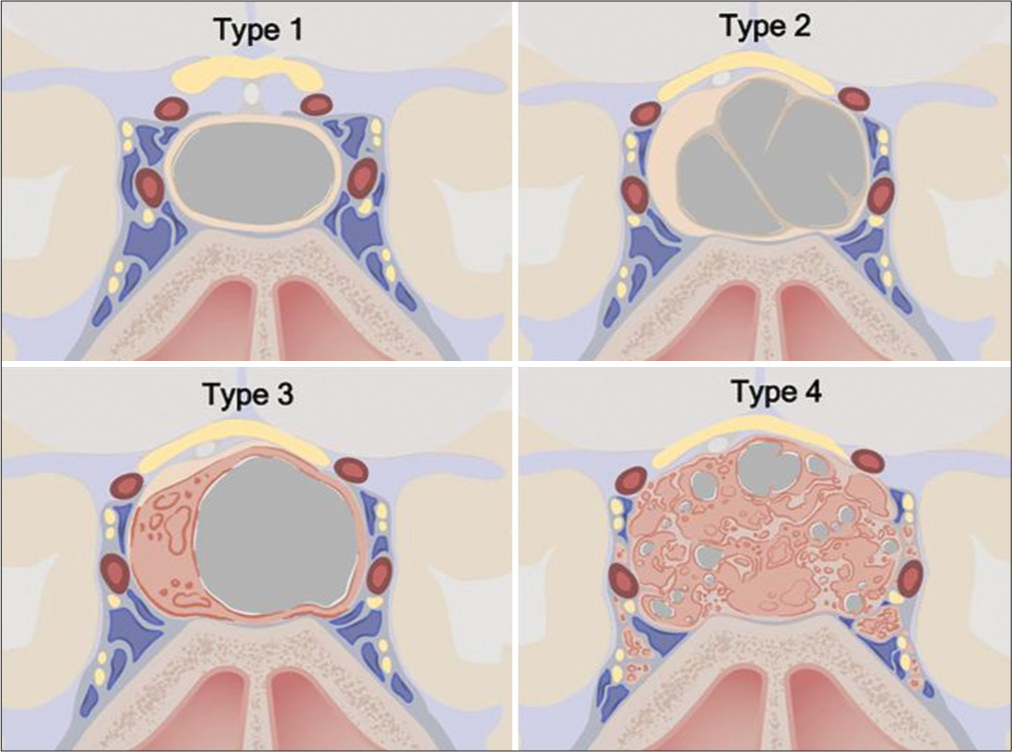
In addition, morphological variables in cystic appearing lesions, such as septation and solid components, are highly inconstant, and a proper guideline for its analysis was unavailable until 2021 when Tavakol et al. published their work on a new classification for cystic lesions in imaging [
Figure 5:
Cystic sellar lesion classification scheme. Type 1: No solid component; well-circumscribed, homogeneous cyst. Type 2: Little or no solid component; irregular cyst, with septations or abnormal walls. Type 3: Obvious solid component; well-circumscribed homogeneous cyst. Type 4: Obvious solid component present; irregular cyst(s) with septations or abnormal walls. Type 1: n = 68 (33.2%), Type 2: n = 72 (35.1%), Type 3: n = 10 (4.9%), Type 4: n = 55 (26.8%). ©2020 Xian Boles, Used by permission.[
This evidence can guide the surgeon to differentiate between the etiologies in sellar cystic lesions, further validation is required. In this case, the conjoined use of these tools positively predicted the final diagnosis of our patient, enabling our team to opt for a proper surgical strategy.
Radiomics
Radiomics features have been described for the radiologic discrimination between cystic lesions in the sellar compartment with optimal performance. Superiority over traditional clinical discrimination goes up to 8% when radiomic methods are employed. Despite this, several limitations still impede the routine use of this technology. There are no prospective studies that demonstrate superiority against traditional diagnostic methods with possible bias arousing from the exclusion of patients who were not treated with surgery. Similarly, external validation with multi-center studies is unavailable.[
CONCLUSION
Effective preoperative differentiation of RCCs from cystic pituitary adenomas can allow the surgeon to opt for a less aggressive approach and avoid specific complications that can result from surgery. Evidence supports the possibility of establishing a preoperative diagnosis for cystic lesions in the sellar region; nevertheless, concomitant use of these tools can increase the odds of defining an accurate diagnosis. Performing prospective studies may help validate and further analyze the conjoined or individual efficacy of these tools and compare their efficacy against emerging technologies.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bonneville F, Cattin F, Bonneville JF, Jacquet G, Viennet G, Dormont D. [Rathke’s cleft cyst]. J Neuroradiol. 2003. 30: 238-48
2. Buchfelder M, Schlaffer S. Surgical treatment of pituitary tumours. Best Pract Res Clin Endocrinol Metab. 2009. 23: 677-92
3. Chotai S, Liu Y, Pan J, Qi S. Characteristics of Rathke’s cleft cyst based on cyst location with a primary focus on recurrence after resection. J Neurosurg. 2015. 122: 1380-9
4. Goel A, Shah A, Jhawar SS, Goel NK. Fluid-fluid level in pituitary tumors: Analysis of management of 106 cases. J Neurosurg. 2010. 112: 1341-6
5. Harrison MJ, Morgello S, Post KD. Epithelial cystic lesions of the sellar and parasellar region: A continuum of ectodermal derivatives?. J Neurosurg. 1994. 80: 1018-25
6. Isono M, Kamida T, Kobayashi H, Shimomura T, Matsuyama J. Clinical features of symptomatic Rathke’s cleft cyst. Clin Neurol Neurosurg. 2001. 103: 96-100
7. Jiang C, Zhang W, Wang H, Jiao Y, Fang Y, Feng F. Machine learning approaches to differentiate sellar-suprasellar cystic lesions on magnetic resonance imaging. Bioengineering (Basel). 2023. 10: 1295
8. Kim E. Symptomatic Rathke cleft cyst: Clinical features and surgical outcomes. World Neurosurg. 2012. 78: 527-34
9. Larkin S, Karavitaki N, Ansorge O, editors. Rathke’s cleft cyst. Handbook of clinical neurology. Netherlands: Elsevier; 2014. 124: 255-69
10. Midha R, Jay V, Smyth HS. Transsphenoidal management of Rathke’s cleft cysts. A clinicopathological review of 10 cases. Surg Neurol. 1991. 35: 446-54
11. Naik VD, Thakore NR. A case of symptomatic Rathke’s cyst. BMJ Case Rep. 2013. 2013: bcr2012006943
12. Park M, Lee SK, Choi J, Kim SH, Kim SH, Shin NY. Differentiation between cystic pituitary adenomas and Rathke cleft cysts: A diagnostic model using MRI. AJNR Am J Neuroradiol. 2015. 36: 1866-73
13. Patyk M, Silicki J, Mazur R, Kręcichwost R, SokołowskaDąbek D, Zaleska-Dorobisz U. Radiomics-the value of the numbers in present and future radiology. Pol J Radiol. 2018. 83: e171-4
14. Ratha V, Patil S, Karmarkar VS, Shah NJ, Deopujari CE. Surgical management of Rathke cleft cysts. World Neurosurg. 2017. 107: 276-84
15. Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: A structured review and meta-analysis. Pituitary. 2012. 15: 71-83
16. Sade B, Albrecht S, Assimakopoulos P, Vézina JL, Mohr G. Management of Rathke’s cleft cysts. Surg Neurol. 2005. 63: 459-66
17. Seo SH, Hwang K, Ji SY, Han JH, Kim CY. Surgical management and long-term results of Rathke’s cleft cyst. J Korean Neurosurg Soc. 2023. 66: 82-9
18. Shatri J, Ahmetgjekaj I. Rathke’s cleft cyst or pituitary apoplexy: A case report and literature review. Open Access Maced J Med Sci. 2018. 6: 544-7
19. Tavakol S, Catalino MP, Cote DJ, Boles X, Laws ER, Bi WL. Cyst type differentiates Rathke cleft cysts from cystic pituitary adenomas. Front Oncol. 2021. 11: 778824
20. Wang SS, Xiao DY, Yu YH, Jing JJ, Zhao L, Wang RM. Diagnostic significance of intracystic nodules on MRI in Rathke’s cleft cyst. Int J Endocrinol. 2012. 2012: 958732
21. Zada G. Rathke cleft cysts: A review of clinical and surgical management. Neurosurg Focus. 2011. 31: E1
22. Zada G, Lin N, Ojerholm E, Ramkissoon S, Laws ER. Craniopharyngioma and other cystic epithelial lesions of the sellar region: A review of clinical, imaging, and histopathological relationships. Neurosurg Focus. 2010. 28: E4
23. Zhang Y, Shang L, Chen C, Ma X, Ou X, Wang J. Machine-learning classifiers in discrimination of lesions located in the anterior skull base. Front Oncol. 2020. 10: 752