- Department of Neurology, Yale School of Medicine, New Haven, Connecticut, United States.
- Pulmonary and Critical Care Section, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, United States.
- Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut, United States.
- Department of Psychiatry, Yale School of Medicine, New Haven, Connecticut, United States.
Correspondence Address:
Jessica Magid-Bernstein, Department of Neurology, Yale School of Medicine, New Haven, Connecticut, United States.
DOI:10.25259/SNI_382_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Christine E. Gummerson1, Melvin Parasram1, Teng J. Peng1, John M. Picard1, Peter A. Kahn2, Evan Angelus3, Shivani Bhatt4, Adam de Havenon1, Adam S. Jasne1, Jessica Magid-Bernstein1. Acute and subacute neurovascular impact of cryptogenic air emboli. 11-Aug-2023;14:285
How to cite this URL: Christine E. Gummerson1, Melvin Parasram1, Teng J. Peng1, John M. Picard1, Peter A. Kahn2, Evan Angelus3, Shivani Bhatt4, Adam de Havenon1, Adam S. Jasne1, Jessica Magid-Bernstein1. Acute and subacute neurovascular impact of cryptogenic air emboli. 11-Aug-2023;14:285. Available from: https://surgicalneurologyint.com/surgicalint-articles/12495/
Abstract
Background: Cerebral air embolism is a rare cause of acute ischemic stroke that is becoming increasingly well-described in the literature. However, the mechanism and severity of this type of injury can vary, with ischemia typically emerging early in the course of care. To the best of our knowledge, delayed ischemia in this setting has not yet been described.
Case Description: A stroke code was called for an unresponsive, hospitalized, 75-year-old man. A computerized tomography (CT) scan of the head revealed air within the right greater than left hemispheric cortical veins with loss of sulcation, concerning for developing ischemia, and CT angiography revealed absent opacification of the distal cortical vessels in the right anterior cerebral artery and middle cerebral artery territories. Magnetic resonance imaging (MRI) of the brain was obtained 5.75 h after the patient’s last known well-showed small areas of subtle cortical diffusion restriction. Follow-up CT head within 24 h showed near-complete resolution of the air emboli after treatment with 100% fraction of inspired oxygen on mechanical ventilation. Subsequent MRI, performed 4 days after the initial event, showed extensive cortical diffusion restriction and cerebral edema crossing vascular territories.
Conclusion: This case highlights that cerebral air emboli can cause delayed ischemia that may not be appreciated on initial imaging. As such, affected patients may require intensive neurocritical care management, close neurologic monitoring, and repeat imaging irrespective of initial radiographic findings.
Keywords: Air embolism, Embolism, Neurocritical care, Neuroimaging, Stroke
CASE REPORT
A 75-year-old man with a history of hypertension, atrial fibrillation on apixaban, stage III chronic kidney disease, peripheral vascular disease, and prior Group B Streptococcal infective endocarditis with prior bioprosthetic valve replacement was admitted to the hospital with recurrent endocarditis and Group B Streptococcus bacteremia. His hospital course was further complicated by right-sided empyema requiring the placement of a chest tube. On hospital day 13, a stroke code was called for unresponsiveness with the last known well 20 min before stroke code activation. On evaluation, the patient was obtunded with forced left gaze deviation concerning for possible nonconvulsive status epilepticus (NCSE). He was intubated for airway protection and treated acutely with intravenous lorazepam and levetiracetam for possible NCSE. Noncontrast computerized tomography (CT) of the head revealed air within the right greater than left hemispheric cortical vessels with loss of sulcation, suggestive of ischemia due to cerebral air embolism (CAE) [
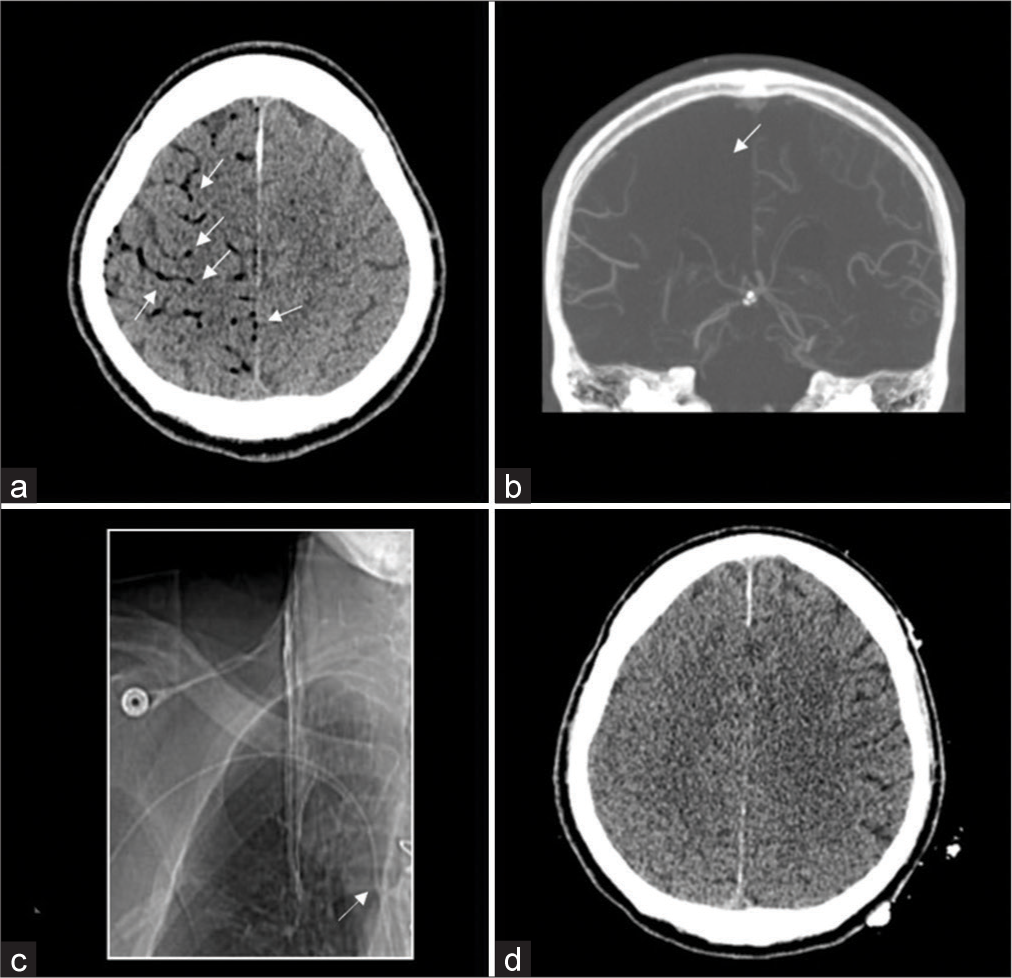
Figure 1:
Initial computerized tomography (CT) demonstrating air emboli (a, white arrows). Loss of blood flow noted in the affected area on CT angiography (CTA) (b, white arrow). CTA scout image showing R-sided peripherally inserted central catheter with correct placement (c, white arrow). Repeat CT within 24 h after initial CT shows improvement in air after 100% fraction of inspired oxygen (d).
Subsequent CT head at 24 hours (h) after the last known well-showed near-complete resolution of the air emboli after continued treatment with 100% FiO2, though there was the evident loss of sulcation in the right hemisphere [
Retrograde movement of air into the cortical veins resulting in venous infarction was the hypothesized mechanism of this patient’s CAE. Although the PICC line was noted to be ipsilateral to the more affected hemisphere, this was confirmed to be in the correct position on CTA scout imaging during the patient’s emergent evaluation and, thus, was not felt to be the source of air entry into the venous circulation [
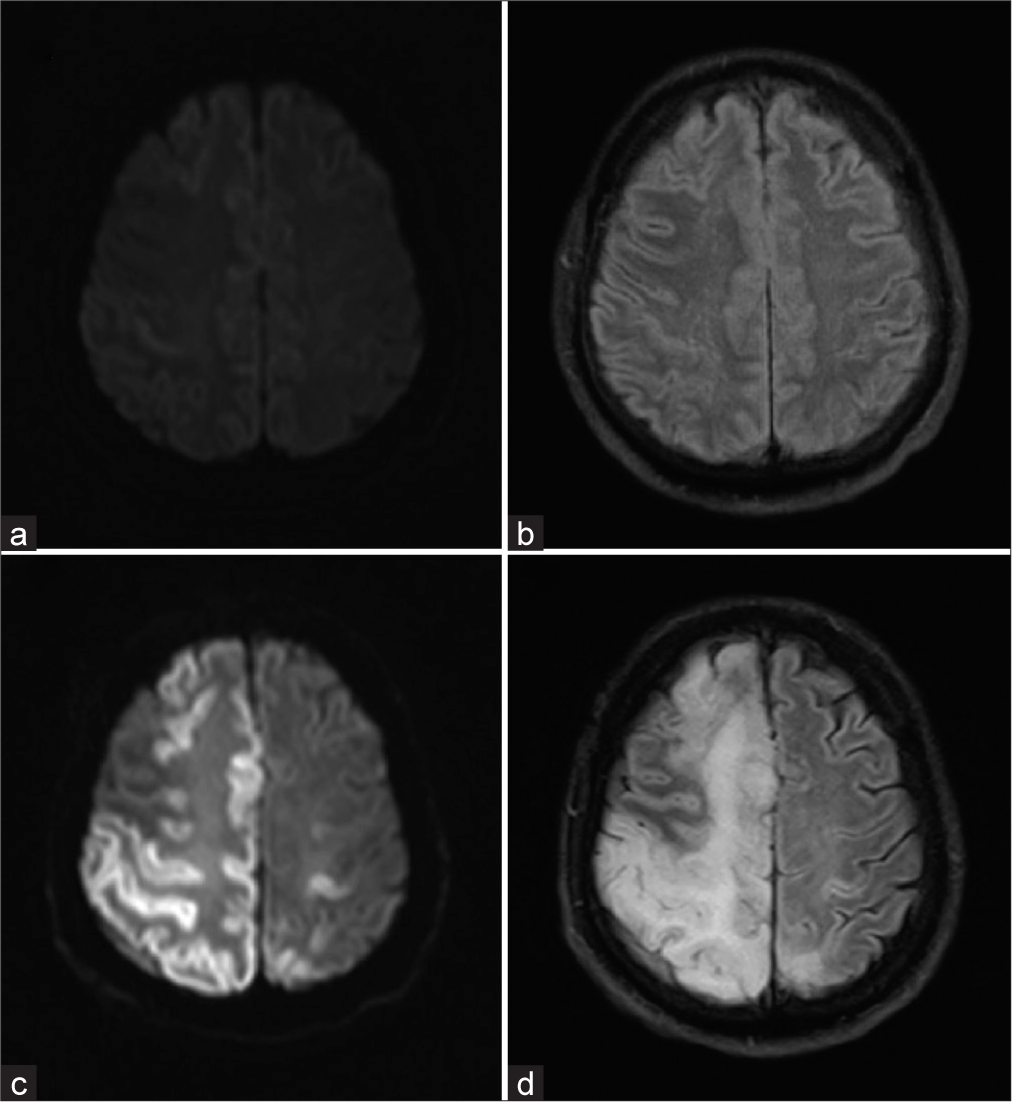
Figure 2:
Axial diffusion-weighted imaging (DWI) (a) and fluid-attenuated inversion recovery (FLAIR) (b) images from magnetic resonance imaging (MRI) brain obtained 5.75 h after last known normal and repeat MRI from day 5 (c: DWI; d: FLAIR), demonstrating delayed evolution of cortical ischemia with adjacent white matter edema.
CAE is a rare cause of acute ischemic stroke that is becoming increasingly well-described in the literature.[
As there are no trials evaluating the treatment of CAE, management strategies are based on case reports and case series. Initial management of CAE involves identification and removal of potential sources of air entry, positioning the patient using the Durant’s maneuver (left lateral decubitus and Trendelenburg position), high-flow oxygenation, and, if available, use of hyperbaric oxygen therapy.[
A unique feature of our case is the radiographic presentation of delayed cerebral ischemia with cerebral edema on MRI associated with CAE. Based on available radiographic data, the cerebral edema and ischemia appear to have developed sometime between the 5.75 h MRI and the 24 h CT head, though the size of the edema appears to have continued to increase between the 24 h imaging and the MRI completed 4 days later. The MRI findings of cortical diffusion restriction with cerebral edema that is out of proportion to the infarct burden suggest that the air emboli were likely in the venous circulation. The mechanism and severity of injury associated with CAE can vary, with ischemia typically emerging early.
To the best of our knowledge, delayed cerebral ischemia secondary to CAE has not yet been described. This has important implications for patient care, as affected patients may require more intensive neurocritical care and closer neuromonitoring than initially anticipated to optimize treatment after CAE. Although the cause of this patient’s air emboli remains cryptogenic at this time, this case highlights that CAE can cause delayed ischemia that may not be appreciated on initial brain imaging.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to thank Dr. Emily Gilmore for her support and guidance in preparing this manuscript. The authors would also like to thank all of the nurses, physicians, advanced practice providers, and patient care associates who cared for this patient.
References
1. Brown AE, Rabinstein AA, Braksick SA. Clinical characteristics, imaging findings, and outcomes of cerebral air embolism. Neurocrit Care. 2023. 38: 158-64
2. Caufield AF, Lansberg MG, Marks MP, Albers GW, Wijman CA. MRI characteristics of cerebral air embolism from a venous source. Neurology. 2006. 66: 945-6
3. Chuang DY, Sundararajan S, Sundararajan VA, Feldman DI, Xiong W. Accidental air embolism. Stroke. 2019. 50: e183-6
4. Lai D, Jovin TG, Jadhav AP. Cortical vein air emboli with gyriform infarcts. JAMA Neurol. 2013. 70: 939-40
5. McCarthy CJ, Behravesh S, Naidu SG, Oklu R. Air embolism: Practical tips for prevention and treatment. J Clin Med. 2016. 5: 93