- Department of Neurosurgery, Toyama University, Toyama, Japan
- Department of Pediatrics, Toyama University, Toyama, Japan.
Correspondence Address:
Emiko Hori, Department of Neurosurgery, Toyama University, Toyama, Japan.
DOI:10.25259/SNI_703_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Emiko Hori1, Takuya Akai1, Kunitaka Maruyama1, Yu Saito2, Hiromichi Taneichi2, Satoshi Kuroda1. Acute subdural hematoma in an infant with a biphasic clinical course and late reduced diffusion. 22-Dec-2023;14:442
How to cite this URL: Emiko Hori1, Takuya Akai1, Kunitaka Maruyama1, Yu Saito2, Hiromichi Taneichi2, Satoshi Kuroda1. Acute subdural hematoma in an infant with a biphasic clinical course and late reduced diffusion. 22-Dec-2023;14:442. Available from: https://surgicalneurologyint.com/surgicalint-articles/12678/
Abstract
Background: Bright tree appearance (BTA) is a characteristic finding on diffusion-weighted magnetic resonance (MR) imaging with transient high intensity in the white matter. BTA is characteristic of infants with acute encephalopathy with biphasic seizures, but it has also been reported in head trauma cases. In this report, we describe an infant case of traumatic brain injury that demonstrated a biphasic clinical course and late reduced diffusion (TBIRD).
Case Description: A 5-month-old boy suffered from head trauma and developed coma and seizures. Computed tomography scans revealed acute subdural hematoma on the right side. He underwent an emergency operation to remove the hematoma but subsequently had seizure clusters for three days. Diffusion-weighted MR imaging revealed BTA in the right cerebral hemisphere. He was treated with antiepileptic agents and fully recovered to pre-injury condition, and MR imaging no further revealed any BTA 20 days after head trauma. He developed no complications at the 10-month postoperative follow-up.
Conclusion: We reported a case of TBIRD following head trauma in the infant. The pathogenesis remains unclear, but we consider the possibility of biphasic seizures in infant head trauma cases, and we should appropriately administer the anticonvulsants and carefully check for MR imaging.
Keywords: Bright tree appearance, Glutamate, Head trauma, Seizure, TBIRD
INTRODUCTION
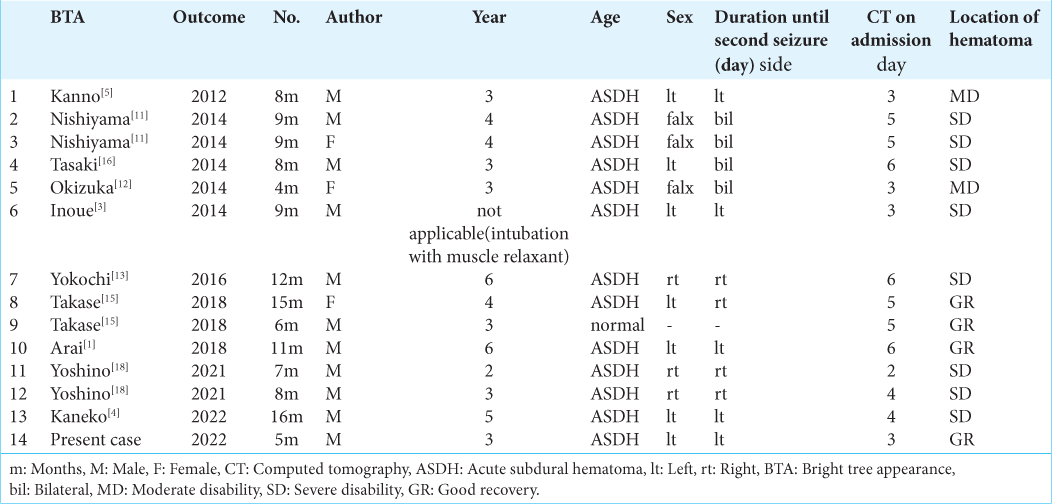
Transient high intensity on the white matter is called as a bright tree appearance (BTA). It is known to be a specific finding on diffusion-weighted magnetic resonance (MR) imaging (DWI). This finding was first reported in infants with acute encephalopathy and was named as biphasic seizures and late reduced diffusion (AESD). AESD occurs in infants younger than two years of age whose myelination is incomplete and who follow biphasic clinical courses. Takase et al. reported two infantile cases of brain injury that developed a cluster of seizures after calming down an early seizure. These cases demonstrated mimicking AESD on DWI. They reviewed seven previously reported cases that showed very similar findings on DWI and called it as an infantile traumatic brain injury with a biphasic clinical course and late reduced diffusion (TBIRD).[
CASE REPORT
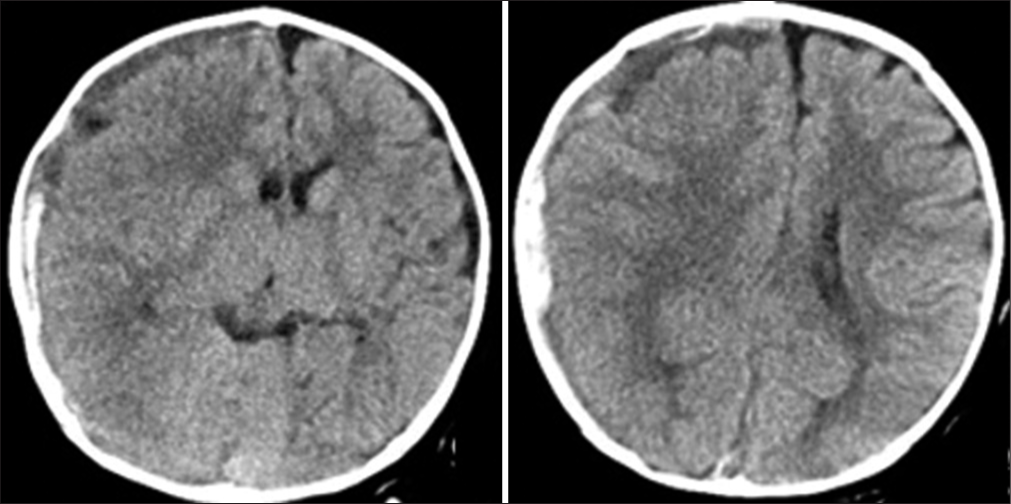
A healthy 5-month-old boy collapsed while sitting up and banged the back of his head. He developed repeated seizures and was transferred to our hospital. Neurological examinations on admission revealed consciousness disturbance (Glasgow Coma Scale, E1V1M5), anisocoria (right/left: 5.0/3.0 mm), and left hemiplegia. Computed tomography (CT) revealed an acute subdural hematoma (ASDH) on the right side with the midline shift [
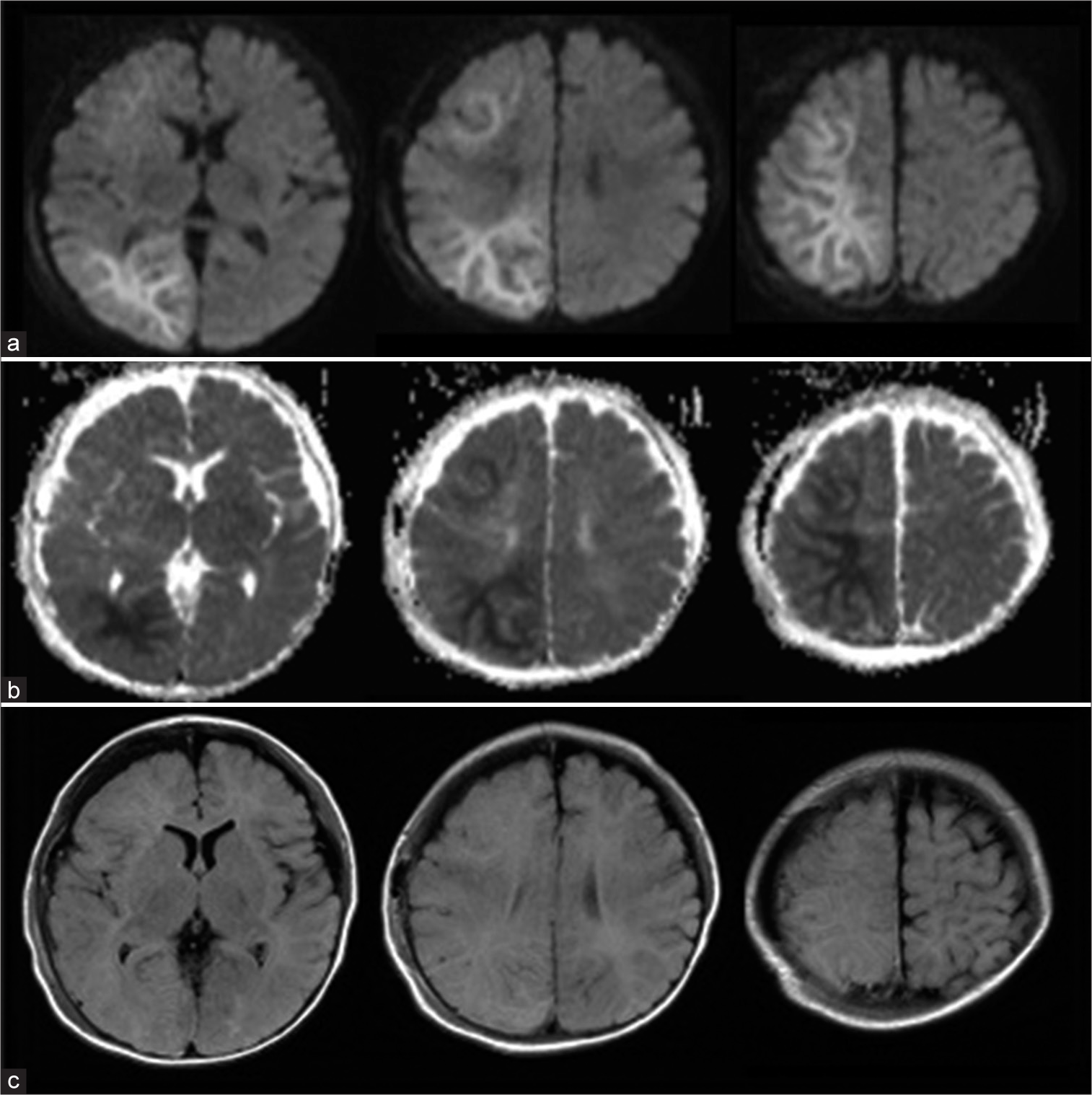
Following surgery, he was neurologically normal and was free from seizures under phenobarbital administration. However, on day 3, he presented with a cluster of seizures in his left upper extremity with consciousness disturbance. MR images revealed a high signal intensity in the white matter of the right frontal and parieto-occipital lobes on DWI, which was consistent with the so-called BTA [
Figure 2:
Magnetic resonance images on day 3 revealed the bright tree appearance on the right frontal and parietal lobes at diffusion-weighted images (DWI). That lesion demonstrated low intensity at the apparent diffusion coefficient map image and iso-intensity at the fluid-attenuated inversion recovery (FLAIR) image. (a) DWI. (b) Apparent diffusion coefficient map image. (c) FLAIR image.
Figure 3:
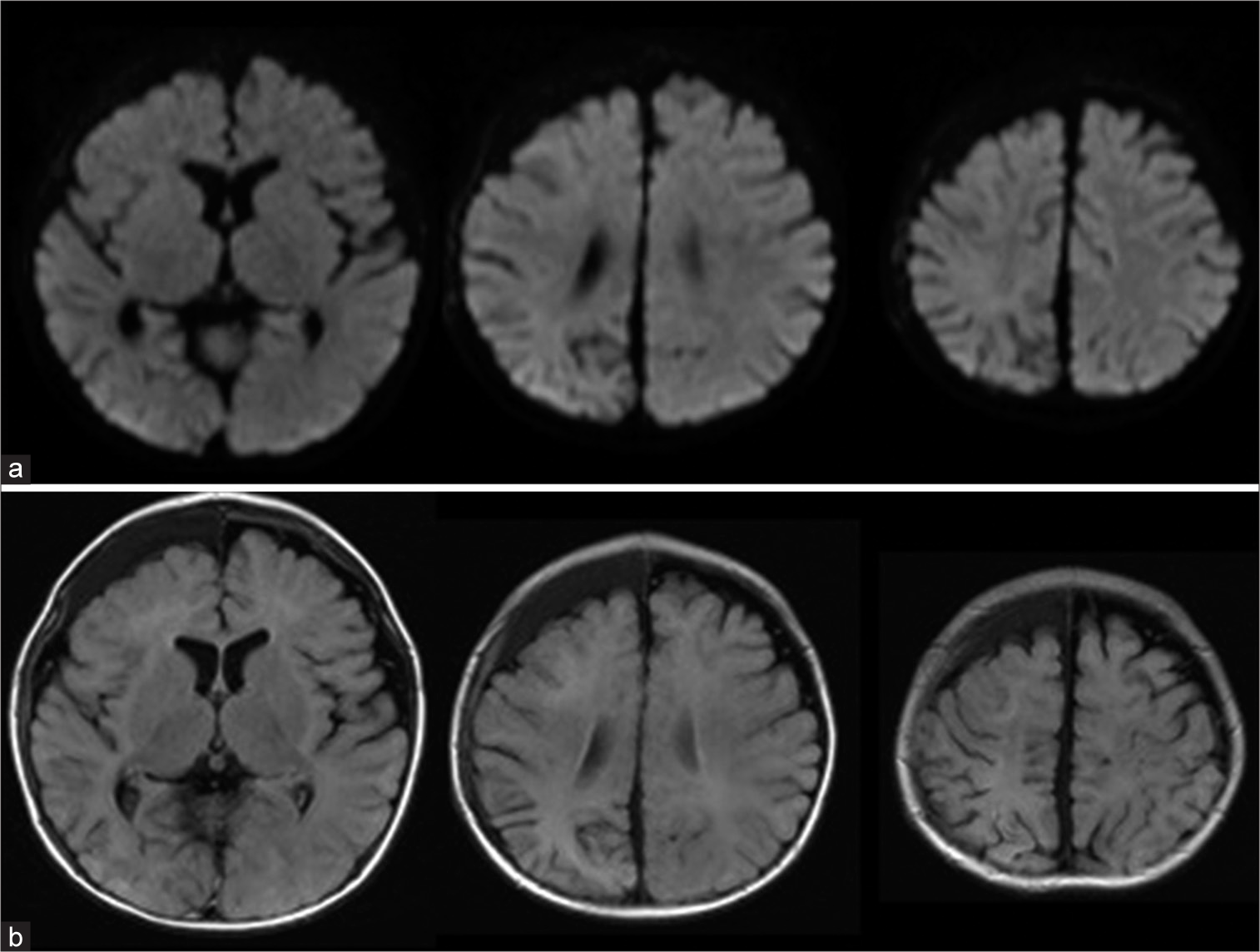
Magnetic resonance images on day 20 showed the abnormal signal intensity in the white matter of the right cerebral hemisphere completely disappeared on diffusion-weighted imaging (DWI), although the high-intensity area in the right parietal lobe with atrophy was observed on fluid-attenuated inversion recovery (FLAIR) images. (a) DWI. (b) FLAIR image.
DISCUSSION
Post-traumatic seizures are more common in children than in adults. Early seizure in traumatic brain injury is reported in 0.4% of patients over 18 years of age,[
Glutamate metabolism may be a factor for TBIRD pathogenesis. In AESD, that clinical course is similar to TBIRD; glutamate increases on days 1–4, and glutamine increases on days 4–12 by MR spectroscopy in the presenting BTA,[
Unfortunately, this case had no glutamate and glutamine measurement by MR spectroscopy. Therefore, although the possibility that damage to the cortical vein affected the seizures might be considered since the MR imaging showed BTA in areas other than the perfusion area of the cortical vein that was the source of the hemorrhage, we considered it likely that elevation of glutamate was affecting the seizures. In this case, even during the nonconvulsive state, EEG revealed spikes and waves. Nonconvulsive seizure due to elevation of glutamate is likely to have continued after the control of the first seizure, and appropriate anticonvulsant administration might prevent a second seizure and white matter damage.
Here, we reported a case of TBIRD in an infant. Infants with ASDH under two years may have a biphasic clinical course, even without brain contusion. We speculated that increased glutamate levels in ASDH may play an important role in this condition. Head trauma cases with early seizures in infants under two years of age should be treated with appropriate anticonvulsants and follow-up MR imaging, including DWI and EEG.
CONCLUSION
Herein, we reported a case of TBIRD with a BTA. The TBIRD is a relatively new concept. The details of its pathogenesis and clinical features are unclear. The excitotoxicity of glutamate, which is increased in subdural hematoma, has been suggested as one of the factors of TBIRD, such as in patients with AESD. Careful attention should be paid to late seizures in cases of head, especially in infants under two years of age with ASDH.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Arai Y, Nikaido K, Koseki N, Takayama R, Watanabe T. Biphasic seizure and unilateral bright tree appearance on diffusion weighted MRI after traumatic brain injury: A case report. Jpn J Pediatr. 2018. 71: 1421-6
2. Elsamadicy AA, Koo AB, David WB, Lee V, Zogg CK, Kundishora AJ. Post-traumatic seizures following pediatric traumatic brain injury. Clin Neurol Neurosurg. 2021. 203: 106556
3. Inoue H, Hasegawa S, Kajimoto M, Matsushige T, Ichiyama T. Traumatic head injury mimicking acute encephalopathy with biphasic seizures and late reduced diffusion. Pediatr Int. 2014. 56: e58-61
4. Kaneko N, Nishizawa H, Fujimoto J, Nanao T, Kimura Y, Owada G. An infantile traumatic brain injury with a bright tree appearance detected before the late seizure. Childs Nerv Syst. 2022. 39: 285-8
5. Kanno A, Komatsu K, Nomura T, Inamura S, Imaizumi T, Kamada J. Suspected shaken baby syndrome; a case report. J Kushiro City Gen Hosp. 2012. 24: 117-20
6. Kierans AS, Kirov II, Gonen O, Haemer G, Nisenbaum E, Babb JS. Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology. 2014. 82: 521-8
7. Koizumi H, Fujisawa H, Kato S, Fujii M, Kajiwara K, Nomura S. Changes in extracellular levels of glutamate in the penumbra zone surrounding a focal traumatic brain lesion: In vivo microdialysis for biochemical monitoring. Neurosurg Emerg. 2008. 13: 37-44
8. Liesemer K, Bratton SL, Zebrack CM, Brockmeyer D, Statler KD. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: Rates, risk factors, and clinical features. J Neurotrauma. 2011. 28: 755-62
9. Majidi S, Makke Y, Ewida A, Sianati B, Qureshi AI, Koubeissi MZ. Prevalence and risk factors for early seizure in patients with traumatic brain injury: Analysis from national trauma data bank. Neurocrit Care. 2017. 27: 90-5
10. Mukoyama T, Moriya T, Miyashita N, Sakurai A, Kinoshita K, Tanjoh K. Usefulness of extracellular glutamate concentration measurement for the postoperative management of patients with acute subdural hematoma: Two case reports. J Jpn Soc Intensive Care Med. 2011. 18: 89-93
11. Nishiyama M, Fujita K, Maruyama A, Nagase H. Two cases of traumatic head injury mimicking acute encephalopathy with biphasic seizures and late reduced diffusion. Brain Dev. 2014. 36: 928-31
12. Okizuka Y, Kawasaki T, Okumura Y, Ito Y, Minamino H, Koizumi T. An infant who suffered abusive head trauma with abnormal signal intensity in the subcortical white matter on diffusion-weighted brain magnetic reonance imaging. J Jpn Pediatr Soc. 2014. 118: 494-9
13. Takanashi J, Mizuguchi M, Terai M, Barkovich AJ. Disrupted glutamate-glutamine cycle in acute encephalopathy with biphasic seizures and late reduced diffusion. Neuroradiology. 2015. 57: 1163-8
14. Takanashi JI, Yasukawa K, Murofushi Y, Masunaga A, Sakuma H, Hayashi M. Loss of myelinated axons and astrocytosis in an autopsy case of acute encephalopathy with biphasic seizures and late reduced diffusion. Brain Dev. 2018. 40: 947-51
15. Takase N, Igarashi N, Taneichi H, Yasukawa K, Honda T, Hamada H. Infantile traumatic brain injury with a biphasic clinical course and late reduced diffusion. J Neurol Sci. 2018. 390: 63-6
16. Tasaki Y, Igarashi N, Kubo T, Shiona S, Fujita S, Hatasaki K. An infant with diffuse brain injury and atrophy after fall: Difficulty in differentiating abuse and trauma. Jpn J Pediatr. 2014. 67: 911-5
17. Yokochi T, Takeuchi T, Mukai J, Ishidou Y, Matsuishi T, Akita Y. Two cases of bright tree appearance on cranial magnetic resonace imaging with diffrent clinical courses from the typical acute encephalopathy with biphasic seizures and late reduced diffusion. J Jpn Soc Emerg Pediatr. 2016. 15: 61-5
18. Yoshino T, Furuya S, Ishikawa J, Fukuoka M, Kim K, Kuki I. Two cases of suspected infantile abusive head trauma who presented symptoms similar to acute encephaloathy with biphasic seizures and late reduced diffusion. J Jpn Soc Emerg Pediatr. 2021. 20: 43-7
19. Zhang H, Zhang X, Zhang T, Chen L. Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin Chem. 2001. 47: 1458-62