- School of Medicine and Health Sciences, Tecnologico de Monterrey, Zapopan, Jalisco, Mexico,
- Department of Neurology, Instituto Neurologico de Guadalajara S.C., Guadalajara, Jalisco, Mexico.
DOI:10.25259/SNI_385_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Carlos Perez-Vega1, Pilar Robles-Lomelin1, Isabel Robles-Lomelin1, Alexandra Diaz-Alba1,2, Victor Garcia Navarro1,2. Acute subdural hematoma recurrence during drain removal associated with spontaneous intracranial hypotension – A non-reported complication. 02-Oct-2020;11:316
How to cite this URL: Carlos Perez-Vega1, Pilar Robles-Lomelin1, Isabel Robles-Lomelin1, Alexandra Diaz-Alba1,2, Victor Garcia Navarro1,2. Acute subdural hematoma recurrence during drain removal associated with spontaneous intracranial hypotension – A non-reported complication. 02-Oct-2020;11:316. Available from: https://surgicalneurologyint.com/surgicalint-articles/10306/
Abstract
Background: Spontaneous intracranial hypotension (SIH) is an uncommon, benign, and generally self-limiting condition caused by low cerebrospinal fluid (CSF) volume and pressure usually caused by a CSF leak. Patients with SIH have an increased incidence of subdural hematomas (SDH), which may be bilateral and recurrent.
Case Description: We report a unique case of a man presenting with SIH and bilateral SDH that were drained with bilateral craniotomies. During drain removal, the patient had an acute neurological deterioration and a CT scan showed SDH recurrence. The patient had two new recurrent SDH afterwards. After the third surgical intervention, the drain was removed in the OR with concomitant subdural saline infusion, there was no recurrence of SDH after that and the patient has had no further complications after a 2-year follow-up.
Conclusion: Patients with intracranial hypotension are predisposed to form SDH. In this case, drain removal caused further decrease in intracranial pressure and triggered a new SDH formation, subdural saline irrigation masked atmospheric pressure and prevented this complication from happening again.
Keywords: Drain removal, Spontaneous intracranial hypotension, Subdural hematomas, Subdural irrigation
INTRODUCTION
Spontaneous intracranial hypotension (SIH) is an uncommon, benign, and generally self-limiting condition caused by low cerebrospinal fluid (CSF) volume and pressure usually caused by a CSF leak.[
CASE REPORT
History of present illness and first intervention
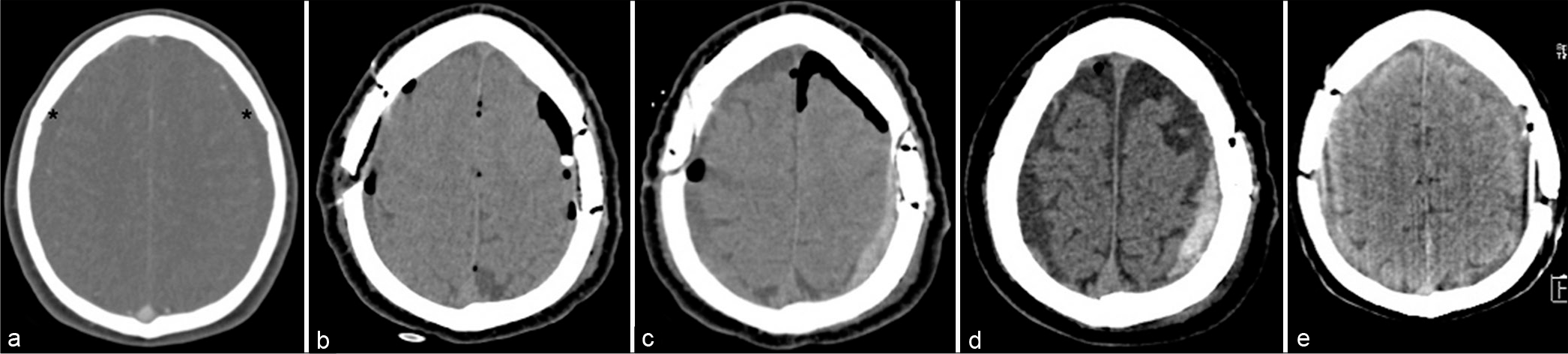
A 48-year-old man presented with an orthostatic headache of 3 months duration that progressively worsened for the past few hours, along with somnolence and vomiting. There was no significant medical history. A noncontrast head CT scan was ordered, showing bilateral subacute SDH [
Figure 1:
CT scans showing the patient’s evolution. (a) Initial CT scan showing bilateral subdural hematomas. *(b) Postoperative CT scan showing bilateral mini-craniotomy with associated pneumoencephalus. (c) Acute left subdural hematoma after the right drain removal. (d) Postoperative CT scan taken after the first recurrence and second surgery, showing the left subdural hematoma that appeared 3 weeks after the second drain removal. (e) Final CT scan, the patient had no further subdural hematoma recurrences.
Drain removal and second intervention
The drain on the right side was removed 24 h after surgery at a bedside procedure; moments afterwards the patient suddenly presented with a headache, vomiting, tonic posture, right ocular deviation, and an altered mental status. A new CT scan showed recurrence of the SDH on the left cerebral hemisphere, contralateral to the drain that was removed [
Diagnosis of SIH
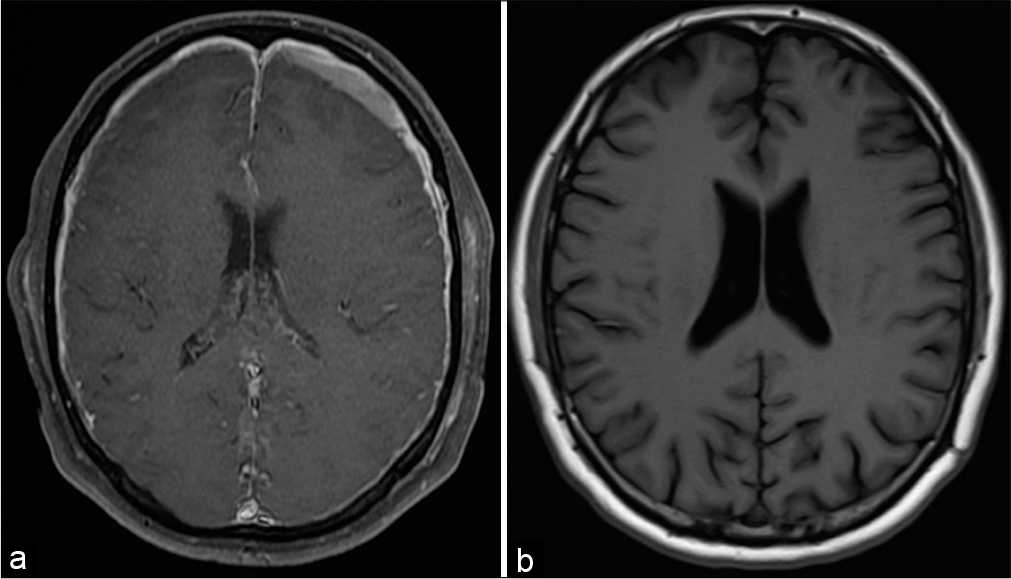
Based on characteristic clinical findings of orthostatic headaches and SDH, a differential diagnosis of SIH was established, and an MRI was performed. The diagnosis was confirmed with classic findings of diffuse pachymeningeal enhancement, enlargement of pituitary gland, engorgement of sigmoid sinus, brainstem sagging and effacement of the suprasellar and prepontine cisterns, as well as a decreased mamillopontine distance. However, a CT myelography did not show findings suggestive of CSF leakage.
Third intervention and remission
Three weeks after the second surgical intervention, the patient developed somnolence, hiccups, positional headache, oculomotor nerve palsy, and respiratory failure requiring assisted mechanical ventilation. An epidural blood patch (EBP) procedure targeting thoracolumbar spinal cord was done, with no change in the clinical outcome. A new CT scan showed recurrence of the left SDH and a new intervention for hematoma drainage with drain placement was scheduled [
Figure 2:
Preoperative and postoperative MRI scans. (a) Gadolinium-enhanced coronal head MRI showing a left subdural hematoma and diffuse pachymeningeal enhancement, taken after the first surgery and drain removal. (b) Control MRI taken 5 months after the third surgery, there is no recurrence of subdural hematoma or signs of intracranial hypotension.
DISCUSSION
SIH is caused by a CSF leak that gives rise to intracranial hypotension; its main clinical manifestation is an orthostatic headache that can be accompanied by other symptoms including nausea, vomiting, neck stiffness, or ocular nerve palsies.[
Acute clinical manifestations triggered by the drain removal in our case show the dual presentation of SIH. The patient presented with somnolence, vomiting, and a severe headache that remitted with hematoma drainage, suggesting a compressive origin of his manifestations. It is hypothesized that drain removal causes a direct communication between the atmosphere and intrathecal space, and the atmosphere pressure further decreases the intracranial pressure in predisposed patients with SIH.[
After the third hematoma drainage, we did a thorough analysis to implement a technique that allowed drain removal without subsequent intracranial hypotension. To avoid further decrease of the intracranial pressure and mask the effect of atmospheric pressure, we infused saline solution into the subdural space simultaneous to drain retraction. The patient did not course with worsening signs and symptoms and has been asymptomatic for 2 years.
It is important to acknowledge that bridging veins can be teared as the drain is removed and subsequently caused a hemorrhage into the subdural space. Although it is a possibility, Alcala-Cerra et al. reported no difference in hematoma recurrence between patients with chronic SDH treated with and without subdural drain after hematoma drainage in seven randomized clinical trials, further raising the question of the relevance of the relationship with bridging veins – drain. SDH recurrence in patients with SIH could represent many etiologies, and the propensity of patients to develop this condition could mean a low resistance threshold for all the possible causes of SDH.[
García-Morales et al. reported a case with four surgical interventions for symptomatic recurrent SDH in a patient with SIH; although no relationship was specified between hematoma recurrences and drain removal, conservative treatment for SIH and subsequent hematoma drainages was the hallmarks of the case.[
CONCLUSION
SIH is a frequently misdiagnosed disorder with a myriad of neurologic signs and symptoms. Patients are prone to develop recurrent SDHs that are difficult to treat. No standardized treatment approach has been established for this type of presentation as some centers perform EBP either before or after hematoma evacuation, if the latter is required. We present a novel approach for the treatment of recurrent SDH in patients with SIH, clinical resolution was achieved with transoperative subdural saline infusion, as the effects of the direct communication of atmospheric pressure and intracranial cavity can be lessened by fluid volume compensation. Further, consensus needs to be reached as the prognosis of these patients can be largely impacted by an approach proven to be safe and free of long-term complications.
Ethics approval
This report was approved by Comite de Bioetica y Cuidados Paliativos HCG-ING Ethics Committee.
Reference number: 2020/0301.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alcala-Cerra G, Young A, Moscote-Salazar LR, PaterninaCaicedo A. Efficacy and safety of subdural drains after burr-hole evacuation of chronic subdural hematomas: Systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 2014. 82: 1148-57
2. Davidson B, Nassiri F, Mansouri A, Badhiwala JH, Witiw CD, Shamji MF. Spontaneous intracranial hypotension: A review and introduction of an algorithm for management. World Neurosurg. 2017. 101: 343-9
3. de Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CA. Subdural haematoma: A potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry. 2003. 74: 752-5
4. Ferrante E, Rubino F, Beretta F, Regna-Gladin C, Ferrante MM. Treatment and outcome of subdural hematoma in patients with spontaneous intracranial hypotension: A report of 35 cases. Acta Neurol Belg. 2018. 118: 61-70
5. García-Morales I, Porta-Etessam J, Galán L, Lagares A, Molina JA. Recurrent subdural haematomas in a patient with spontaneous intracranial hypotension. Cephalalgia. 2001. 21: 703-5
6. Kelley GR, Johnson PL. Sinking brain syndrome: Craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology. 2004. 62: 157
7. Kim JH, Kim JH, Kwon TH, Chotai S. Brain herniation induced by drainage of subdural hematoma in spontaneous intracranial hypotension. Asian J Neurosurg. 2013. 8: 112-5
8. Kim JH, Roh H, Yoon WK, Kwon TH, Chong K, Hwang SY. Clinical features of patients with spontaneous intracranial hypotension complicated with bilateral subdural fluid collections. Headache. 2019. 59: 775-86
9. Michali-Stolarska M, Bladowska J, Stolarski M, Sąsiadek MJ. Diagnostic imaging and clinical features of intracranial hypotension-review of literature. Pol J Radiol. 2018. 83: e11-8
10. Perez-Vega C, Robles-Lomelin P, Robles-Lomelin I, Navarro VG. Spontaneous intracranial hypotension: Key features for a frequently misdiagnosed disorder. Neurol Sci. 2020. 41: 2433-41
11. Schievink WI, Jean-Pierre S, Maya MM, Moser FG, Nuño M. Coma: A serious complication of spontaneous intracranial hypotension. Neurology. 2018. 90: e1638-45
12. Takahashi K, Mima T, Akiba Y. Chronic subdural hematoma associated with spontaneous intracranial hypotension: Therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo). 2006. 56: 69-76
13. Urbach H. Intracranial hypotension: Clinical presentation, imaging findings, and imaging-guided therapy. Curr Opin Neurol. 2014. 27: 414-24
14. Wald JT, Diehn FE. Spontaneous intracranial hypotension. Appl Radiol. 2018. 47: 18-22
15. Yoon SH, Chung YS, Yoon BW, Kim JE, Paek SH, Kim DG. Clinical experiences with spontaneous intracranial hypotension: A proposal of a diagnostic approach and treatment. Clin Neurol Neurosurg. 2011. 113: 373-9