- Associate Professor of Neurosurgery, Department of Neurosurgery, Functional NeuroOncology and Epilepsy Surgery Multidisciplinary Board, CMN Siglo XXI, IMSS, Mexico City, Mexico
- Department of Neurosurgery and Neurotechnology, Universitätsklinik Tübingen, Tübingen, Germany
- Department of Neurosurgery, Stanford University School of Medicine, Palo Alto, United States
- Department of Neurosurgery, University Hospital Bochum, Bochum, Nordrhein-Westfalen, Germany
- Department of Neurosurgery, University of Miami, Miami, United States
- Department of Neurosurgery, Functional Neurosurgery Clinic, CMN Siglo XXI, IMSS, Mexico City, Mexico
- Department of Neurosurgery, Michigan Advanced Pain and Spine, Warren, United States,
- Department of Radiosurgery, Functional and Stereotactic Neurosurgery, General Hospital of Mexico, Mexico City, Mexico.
Correspondence Address:
Bayron A. Sandoval-Bonilla M.D. M.Sc, Associate Professor of Neurosurgery, Department of Neurosurgery, Functional NeuroOncology and Epilepsy Surgery Multidisciplinary Board, CMN Siglo XXI, IMSS, Mexico City, Mexico.
DOI:10.25259/SNI_911_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bayron Alexander Sandoval-Bonilla1, María Fabiola De la Cerda Vargas2, Maximiliano Alberto Nuñez3, Yaroslav Parpaley4, Joacir Graciolli Codeiro5, Jesus Fonseca Cosio6, Ramiro Antonio Perez de la Torre7, Luis Garcia-Muñoz8. Adequate control of seizures in a case of lead migration and neuromodulation of the posterior Sylvian junction: A case report. 05-Apr-2024;15:124
How to cite this URL: Bayron Alexander Sandoval-Bonilla1, María Fabiola De la Cerda Vargas2, Maximiliano Alberto Nuñez3, Yaroslav Parpaley4, Joacir Graciolli Codeiro5, Jesus Fonseca Cosio6, Ramiro Antonio Perez de la Torre7, Luis Garcia-Muñoz8. Adequate control of seizures in a case of lead migration and neuromodulation of the posterior Sylvian junction: A case report. 05-Apr-2024;15:124. Available from: https://surgicalneurologyint.com/surgicalint-articles/12839/
Abstract
Background: This report aims to describe the neuromodulation effect on seizure control in a patient with a left hippocampal migrated electrode to the Posterior Sylvian Junction (PSJ) during a follow-up of 17 years.
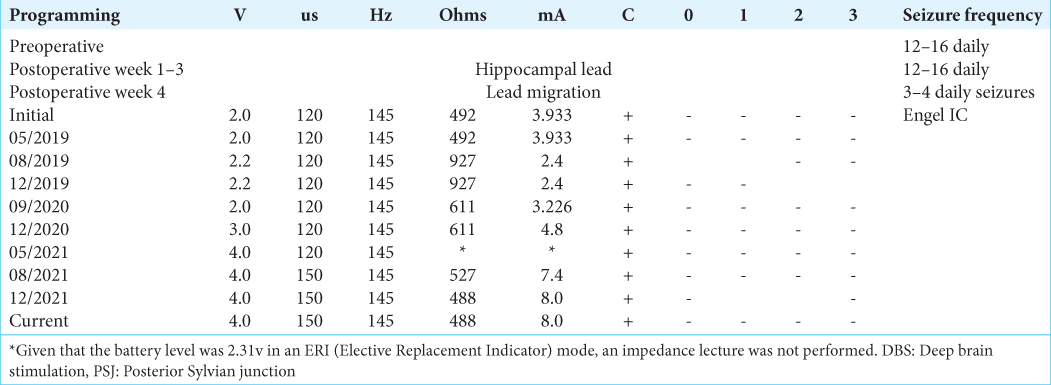
Case Description: We report a case of a female patient with drug-resistant epilepsy who initiated at seven years old and underwent a stereotactic frame-based insertion of a left hippocampal electrode for deep brain stimulation (DBS). Posterior migration of the electrode was identified at PSJ by postoperative magnetic resonance imaging one month after surgery. A consistent seizure reduction (Engel IC) was obtained with 2v-120 uS-145 Hz, contacts 0–3 negative, casing positive DBS parameters and maintained to this day. Patient data were collected from electronic medical records preceded by obtaining an informed consent for research and publication purposes. Stimulation parameter adjustments were confirmed with the digital records of the local device provider (Medtronic).
Results: PSJ is a connectivity confluence point of white matter pathways in the posterior quadrant of the hemispheres. White mater DBS could be considered for research as a potential complementary target for neuromodulation of refractory epilepsy.
Keywords: Case report, Deep brain stimulation, Epilepsy, Posterior Sylvian junction
INTRODUCTION
Drug-resistant epilepsy patients who are not candidates for resective or ablative procedures are consistently evaluated for neuromodulation. Deep brain stimulation (DBS) is an open-loop approach that mostly delivers continuous stimulation to the anterior thalamic nucleus (ANT), the centromedian nucleus of the thalamus (CMT), and the hippocampus (Hipp). Responsive neurostimulation (RNS), on the other hand, is a closed-loop approach with on-demand stimulation at the epileptogenic zone and vicinities with the purpose of abating the ongoing ictal discharges. Both types of stimulation may be delivered through deep seating electrodes covering gray and/or white matter.[
From an anatomical and functional point-of-view, there are identifiable regions of connectivity confluence in the brain where white matter stimulation (WMS) could play a role in seizure control. As an example, WMS of the temporal stem (TS) has been described for bilateral mesial temporal epilepsy (MTLE) with satisfactory results.[
MATERIALS AND METHODS
Patient data were collected from electronic medical records preceded by obtaining an informed consent for research and publication purposes. Stimulation parameter adjustments were confirmed with the digital records of the local device provider (Medtronic). This case report follows the CARE Guidelines.[
CASE REPORT
We report a case of a female patient with drug-resistant epilepsy that initiated at seven years old with frequent motor (focal to bilateral tonic-clonic seizures) and non-motor (impaired awareness seizures) focal onset seizures (12–16 daily seizures), diagnosed, and managed as temporal epilepsy in a pediatric center with no previous medical, family, psychosocial history, surgeries, or any other risks factors. Psycho-affective (fear and anxiety) and visual auras with no oroalimentary or gestural automatisms were also identified during childhood.
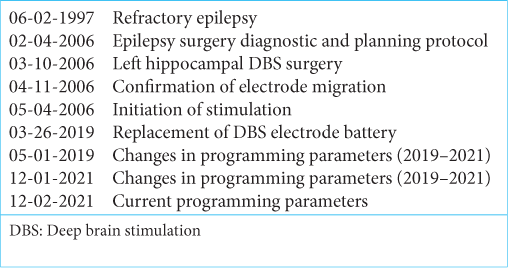
In 2006, at the age of 16 years, the patient was admitted to our center for evaluation. Drug-resistant epilepsy was confirmed (valproate 60 mg/kg/day, levetiracetam 3 g/day, lamotrigine 200 mg/day, and clonazepam 4 mg/day). Examination demonstrated no neurological deficits. Neuropsychological evaluation revealed no memory impairment. Magnetic resonance imaging (MRI) showed left hippocampal atrophy and no structural lesion. Initial interictal electroencephalogram presented with bilateral frontotemporal activity. Video electroencephalogram (vEEG) revealed ictal left temporal epileptiform discharges and generalized interictal slow theta-alpha activity. Given that a consistent elementary visual phenomena (phosphenes) aura was identified during childhood, an invasive intracranial recording was offered to rule out a posterior extratemporal origin. The proposed invasive vEEG with subdural grid implantation to identify an ictal onset zone was not consented to by the family and the patient. For that reason, the patient was considered to be treated as a left temporal refractory epilepsy and a left trans-Sylvian selective amygdalohippocampectomy was offered, but the patient and her family stated the preference for a minimally invasive procedure.
She underwent a stereotactic frame-based insertion (F.L. Fischer, Freiburg, Germany) of a 3387 wide-spaced four contacts deep electrode (DBS Medtronic, Minneapolis, MN, USA) throughout the major axis of the hippocampus using a posterior longitudinal trajectory in February 2006 [
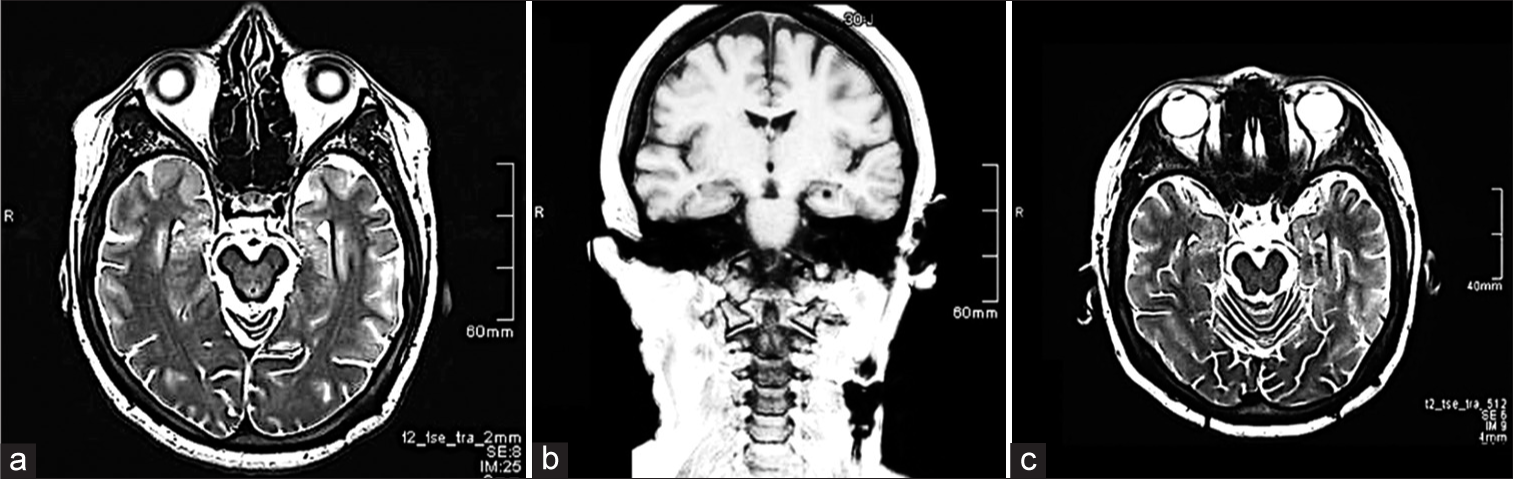
Figure 1:
(a) Preoperative T2-magnetic resonance imaging (MRI), axial view showing left hippocampal atrophy and increased diameter of the temporal horn with no evidence of structural lesion. (b and c) Postoperative MRI, T1-coronal, and T2 axial view, showing electrode position in the left hippocampus.
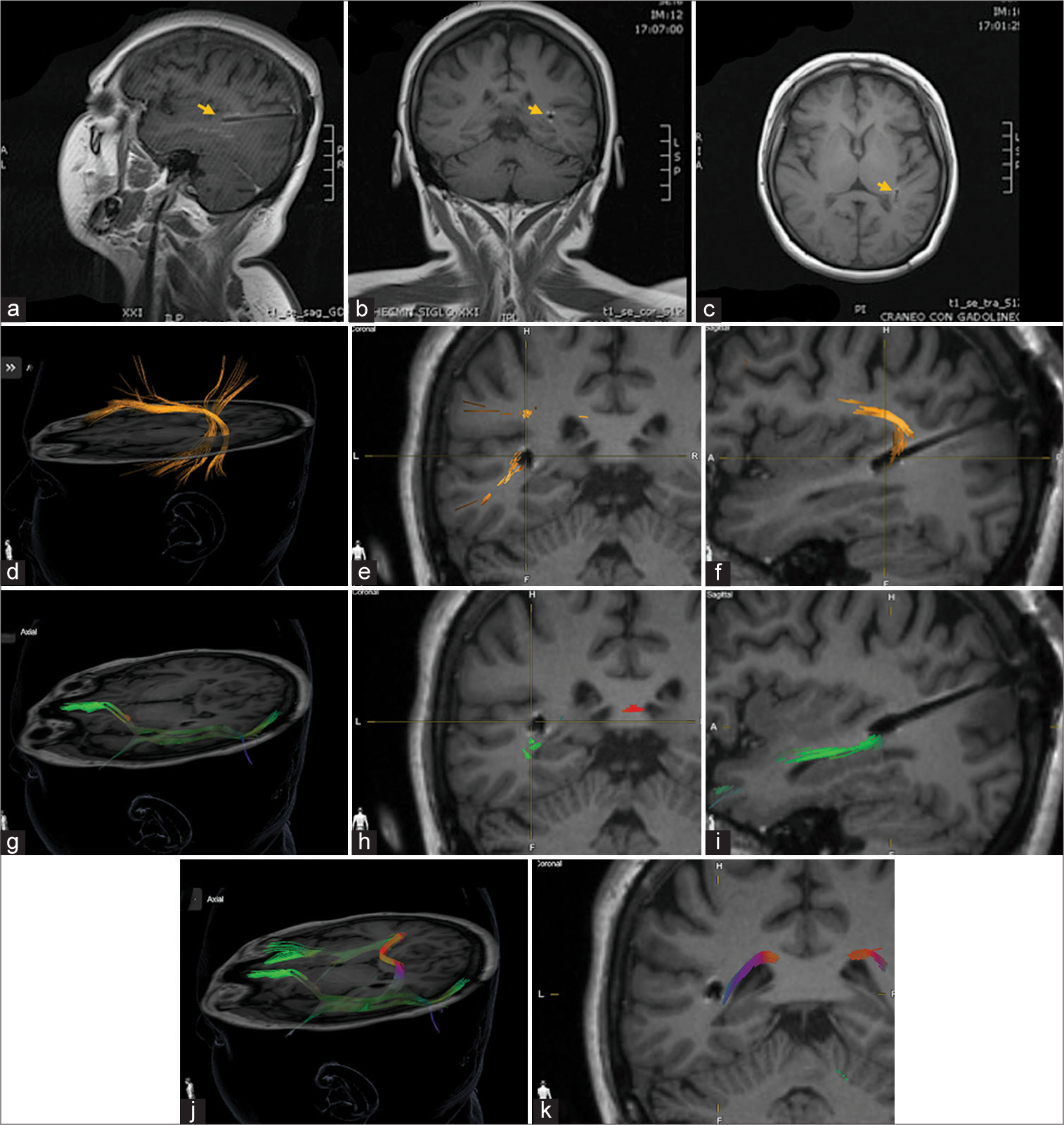
Figure 2:
(a-i) Postoperative magnetic resonance imaging electrode localization and diffuse tensor imaging (DTI) tractrographic reconstruction of Posterior Sylvian Junction (PSJ) (yellow arrow figures a/b/c). (d/e/f) Arcuate-Superior Longitudinal Fascicle (in orange) complex diffusion tensor imaging in axial, coronal, and sagittal view demonstrates the medial position of the electrode in relation to the superficial layer of the PSJ at this level. (g/h/i/j) Inferior Frontal Occipital Fascicle (IFOF) (in green) DTI located in the deep sagittal stratum layer of the PSJ. The electrode is visualized in this layer. (h/j/k) The Tapetum (in red and purple) is identified in the deep layer of the PSJ, medial to the electrode.
Both clinicians and the patient noticed a maintained seizure reduction during a long-term follow-up. In 2019, an invasive recording with SEEG implantation to change and optimize electrode position was proposed to confirm or rule out a temporal or posterior quadrant origin of seizures. However, further invasive evaluation was not consented by the patient and family due to favorable clinical results obtained with the previous PSJ stimulation. From 2019 to 2021, parameters were modified to improve seizure control. Nevertheless, the most favorable outcome (Engel IC) was obtained with original parameters (2v-120 uS-145 Hz, contacts 0–3 negative, casing positive) and maintained to this day [
DISCUSSION
To the best of our knowledge, this is the first case that describes WMS in the posterior region of the hemisphere with a favorable outcome.[
The rationale for neurostimulation in epilepsy is the modulation of a pathologic neural network. DBS for seizure control in refractory epilepsy has been focused on the anterior thalamic nucleus (ANT), CMT, and hippocampus (Hipp).[
As mentioned before, WMS has been described in the TS for bilateral MTLE with RNS.[
Connectivity confluence of epileptic circuits can be established in deep gray nuclei or white matter. Given that the confluence of an specific white matter region where tracts are densely located in compact groups (TS) has been previously targeted for WMS, considering other networks for DBS is feasible. Although further studies are required to confirm an advantage among open or closed-loop approaches for WMS in terms of seizure control and quality of life, propagation of seizures from the mesial and anterior temporal lobe can be controlled with WMS of the anterior segment of the TS.[
Posterior Sylvian Junction
The PSJ is a region of white matter tracts located in the posterior quadrant of the hemisphere defined as the junction of the temporal, occipital, and parietal lobes (PTO junction) with no clear boundaries among them. The PSJ is represented by three layers of white matter tracts from superficial to deep: the superior longitudinal fasciculus complex, the sagittal stratum (SS), and the tapetum. Anteriorly, the PSJ is limited by the insula at the level of the superior and inferior limiting sulcus junction. Posteriorly, it is limited by the caudal end of the occipital horn.
The region between the arcuate/superior longitudinal fasciculus complex (A/SLF) and the tapetum has been traditionally described as SS. From superficial to deep, the middle/inferior longitudinal fascicles, the inferior fronto-occipital fascicle (IFOF), and the optic radiation (OR)/posterior extension of the anterior commissure fibers complex can be identified.[
Propagation of seizures in the posterior part of each hemisphere includes not only the SS but also the superficial and deep white matter fibers surrounding them (A/SLF complex and tapetum). As described before, these regions are continuous and from a connectivity standpoint, we propose to describe them altogether as PSJ. A Klingler’s dissection technique of a left cerebral hemisphere performed in Stanford microsurgical laboratory illustrates the anatomy of the region [
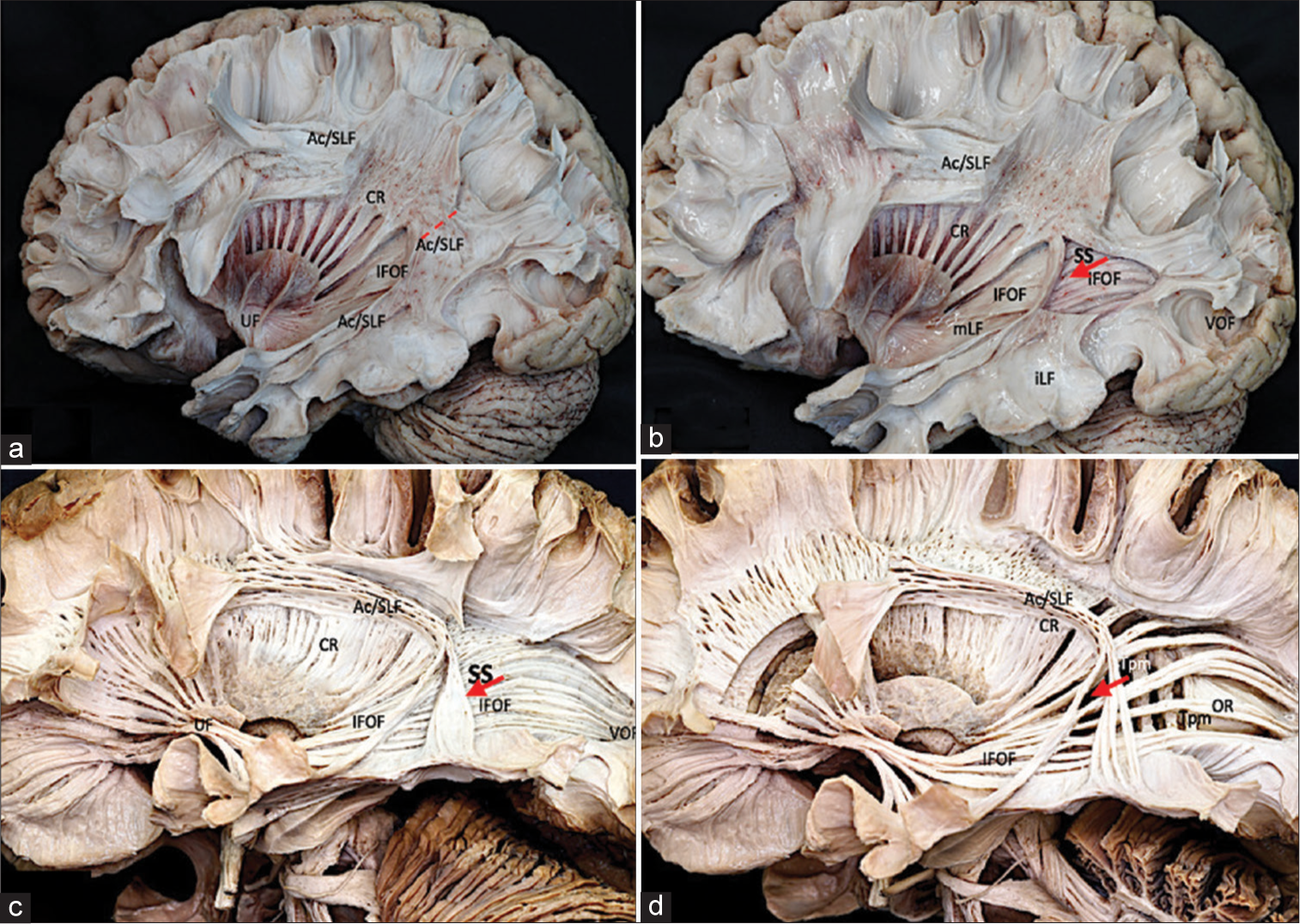
Figure 3:
Anatomical Klingler’s technique dissection of PSJ. (a) Superficial layer: AC/SLF complex. The superficial layer of the PSJ is the Ac/SLF complex, whose fibers have an oblique and vertical disposition in the region the (The dotted red line represents the position of the electrode, medial to Ac/SLF). (b) Superficial sagittal stratum: Both the iLF that is beneath the level of the temporal horn in the inferior portion of the PSJ connecting the anterior temporal region with the occipital lobe and the mLF located under the transverse temporal gyrus connecting the superior temporal gyrus with the inferior parietal lobe represent the next layer of the region. The mLF intersects with the deepest IFOF at the inferior limiting sulcus of the insula. The CR and the VOF are rostral and caudal to the stratum sagitale region. (c) Deep sagittal stratum: The IFOF is the next layer of the PSJ. It originates in the frontal lobe and runs in the external capsule under the anterior and inferior limiting sulcus of the insula toward the posterior part of the occipital and parietal lobes by a superior branch that extends above the atrium and by an inferior branch located in the roof of the temporal horn ending at the posterior temporal and occipital lobes. The initial segment of the OR runs deep to the IFOF both integrated in the posterior thalamic peduncle with the posterior branch of the anterior commissure and the auditory radiations. The optic radiation (OR) originates in the lateral geniculate body and ends in the calcarine fissure running through the roof of the temporal horn and the lateral wall of the atrium and occipital horn. (d) Deep layer: Tapetum. The Tapetum represents the deepest layer of the PSJ and separates the region from the ventricular ependyma connecting the posterior component of both hemispheres (The red arrow (b-d) represents the electrode position, in the PSJ superior to IFOF. The Tapetum presents a medial localization in relation to the electrode position). Ac/SLF: Arcuate-superior longitudinal fascicle complex, CR: Corona radiata, IFOF: Inferior fronto-occipital fascicle, UF: Uncinate fascicle, VOF: Vertical occipital fascicle, mLF: Medial longitudinal fascicle, iLF: Inferior longitudinal fascicle, SS: Stratum Sagitale, Tpm: Tapetum, PSJ: Posterior Sylvian junction.
DBS of PSJ, as described in this case, has maintained adequate seizure control with improvement in quality of life and a positive impact on the perception of the patient about the treatment during a long-term follow-up. As a result of an apparent initial local micro lesioning effect associated with lead migration during the 4th week postimplantation, DBS was initiated, and a significant and sustained seizure reduction during 17 years was obtained. We hypothesize that PSJ requires an optimal stimulation point for greater efficiency in neuromodulation.[
The purpose of reporting this case is to identify that the PSJ is a connectivity confluence point of white-matter pathways in the posterior quadrant of the hemispheres and could be considered for research as a potential complementary target for neuromodulation.
The principal limitation of this case is that we do not have previous SEEG evaluations. Invasive studies were not consented to by the patient and family due to favorable clinical results obtained with the previous PSJ electrode stimulation.
CONCLUSION
Although absolute conclusions cannot be generalized from individual cases and more extensive series are required to confirm or discard the clinical findings reported, we have the hypothesis that connectivity confluence points of the brain are potential complementary targets for DBS of drug-resistant epilepsies. The rationale to consider the functional anatomy of PSJ as a candidate for DBS requires to be proved or rejected by following a prospectively designed protocol. This report is one of the few published cases of WMS for epilepsy.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Cendejas Zaragoza L, Byrne RW, Rossi MA. Pre-implant modeling of depth lead placement in white matter for maximizing the extent of cortical activation during direct neurostimulation therapy. Neurol Res. 2017. 39: 198-211
2. Cukiert A, Cukiert CM, Burattini JA, Mariani PP, Bezerra DF. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: A prospective, controlled, randomized, double-blind study. Epilepsia. 2017. 58: 1728-33
3. Di Carlo DT, Benedetto N, Duffau H, Cagnazzo F, Weiss A, Castagna M. Microsurgical anatomy of the sagittal stratum. Acta Neurochir (Wien). 2019. 161: 2319-27
4. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010. 51: 899-908
5. Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017. 58: 994-1004
6. George DD, Ojemann SG, Drees C, Thompson JA. Stimulation mapping using stereoelectroencephalography: Current and future directions. Front Neurol. 2020. 11: 320
7. Girgis F, Miller JP. White matter stimulation for the treatment of epilepsy. Seizure. 2016. 37: 28-31
8. Maldonado IL, Destrieux C, Ribas EC, de Abreu Brito Guimaraes BS, Cruz PP, Duffau H. Composition and organization of the sagittal stratum in the human brain: A fiber dissection study. J Neurosurg. 2021. 135: 1214-22
9. Nunna RS, Borghei A, Brahimaj BC, Lynn F, Garibay-Pulido D, Byrne RW. Responsive neurostimulation of the mesial temporal white matter in bilateral temporal lobe epilepsy. Neurosurgery. 2021. 88: 261-7
10. Piper RJ, Richardson RM, Worrell G, Carmichael DW, Baldeweg T, Litt B. Towards network-guided neuromodulation for epilepsy. Brain. 2022. 145: 3347-62
11. Ribas EC, Yagmurlu K, de Oliveira E, Ribas GC, Rhoton A. Microsurgical anatomy of the central core of the brain. J Neurosurg. 2018. 129: 752-69
12. Ribas EC, Yagmurlu K, Wen HT, Rhoton AL. Microsurgical anatomy of the inferior limiting insular sulcus and the temporal stem. J Neurosurg. 2015. 122: 1263-73
13. Riley DS, Barber MS, Kienle GS, Aronson JK, von SchoenAngerer T, Tugwell P. CARE guidelines for case reports: Explanation and elaboration document. J Clin Epidemiol. 2017. 89: 218-35
14. Rossi MA, Stebbins G, Murphy C, Greene D, Brinker S, Sarcu D. Predicting white matter targets for direct neurostimulation therapy. Epilepsy Res. 2010. 91: 176-86
15. Salanova V, Sperling MR, Gross RE, Irwin CP, Vollhaber JA, Giftakis JE. The SANTE study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021. 62: 1306-17
16. Valentin A, Anderson M, Alarcon G, Seoane JJ, Selway R, Binnie CD. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain. 2002. 125: 1709-18
17. Velasco AL, Velasco F, Velasco M, Trejo D, Castro G, CarrilloRuiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: A double-blind, long-term follow-up study. Epilepsia. 2007. 48: 1895-903
18. Velasco F, Velasco M, Jimenez F, Velasco AL, Brito F, Rise M. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000. 47: 295-304
19. Vetkas A, Fomenko A, Germann J, Sarica C, Iorio-Morin C, Samuel N. Deep brain stimulation targets in epilepsy: Systematic review and meta-analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia. 2022. 63: 513-24
20. Woolnough O, Donos C, Murphy E, Rollo PS, Roccaforte ZJ, Dehaene S. Spatiotemporally distributed frontotemporal networks for sentence reading. Proc Natl Acad Sci U S A. 2023. 120: e2300252120
21. Zillgitt AJ, Haykal MA, Chehab A, Staudt MD. Centromedian thalamic neuromodulation for the treatment of idiopathic generalized epilepsy. Front Hum Neurosci. 2022. 16: 907716