- Department of Neuroscience, Winthrop Neuroscience, Winthrop University Hospital, Mineola, NY 11501, USA
Correspondence Address:
Nancy E. Epstein
Department of Neuroscience, Winthrop Neuroscience, Winthrop University Hospital, Mineola, NY 11501, USA
DOI:10.4103/2152-7806.170432
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Epstein NE. Adjacent level disease following lumbar spine surgery: A review. Surg Neurol Int 25-Nov-2015;6:
How to cite this URL: Epstein NE. Adjacent level disease following lumbar spine surgery: A review. Surg Neurol Int 25-Nov-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/adjacent-level-disease-following-lumbar-spine-surgery-a-review/
Abstract
Background:Instrumented lumbar spine surgery is associated with an increased risk of adjacent segment disease (ASD). Multiple studies have explored the various risk factors contributing to ASD that include; fusion length (especially, three or more levels), sagittal malalignment, facet injury, advanced age, and prior cephalad degenerative disease.
Methods:In this selective review of ASD, following predominantly instrumented fusions for lumbar degenerative disease, patients typically underwent open versus minimally invasive surgery (MIS), transforaminal lumbar interbody fusions (TLIFs), posterior lumbar interbody fusions (PLIFs), or rarely posterolateral lumbar instrumented or noninstrumented fusions (posterolateral lumbar fusion).
Results:The incidence of ASD, following open or MI lumbar instrumented fusions, ranged up to 30%; notably, the addition of instrumentation in different series did not correlate with improved outcomes. Alternatively, in one series, at 164 postoperative months, noninstrumented lumbar fusions reduced the incidence of ASD to 5.6% versus 18.5% for ASD performed with instrumentation. Of interest, dynamic instrumented/stabilization techniques did not protect patients from ASD. Furthermore, in a series of 513 MIS TLIF, there was a 15.6% incidence of perioperative complications that included; a 5.1% frequency of durotomy and a 2.3% instrumentation failure rate.
Conclusions:The incidence of postoperative ASD (up to 30%) is greater following either open or MIS instrumented lumbar fusions (e.g., TLIF/PLIF), while decompressions with noninstrumented fusions led to a much smaller 5.6% risk of ASD. Other findings included: MIS instrumented fusions contributed to higher perioperative complication rates, and dynamic stabilization did not protect against ASD.
Keywords: Adjacent segment disease, lumbar fusions: Transforaminal lumbar interbody fusions, posterior lumbar interbody fusions: Dynamic stabilization
INTRODUCTION
Performing lumbar spine surgery involving instrumentation increases the risk of adjacent segment disease (ASD). These operations typically include decompressions accompanied by open versus minimally invasive surgery/minimally invasive (MIS/MI) transforaminal lumbar interbody fusions (TLIFs), posterior lumbar interbody fusions (PLIFs); only rarely, are posterolateral lumbar instrumented or noninstrumented fusions (posterolateral lumbar fusion [PLF]) performed. In one series, frequencies of ASD utilizing open versus MIS instrumented fusion techniques ranged up to 30%; in another series, ASD at 164 postoperative months occurred in 18.5% with instrumentation (PLF) versus a substantially lower 5.6% for noninstrumented posterior fusions (PLF).[
Several risk factors contribute to ASD following the application of instrumentation; fusion length (especially three or more levels), preoperative sagittal malalignment, facet injury/tropism, advanced age, increased body mass index (BMI), and preoperative documentation of cephalad degenerative disease (e.g., disc disease, stenosis).[
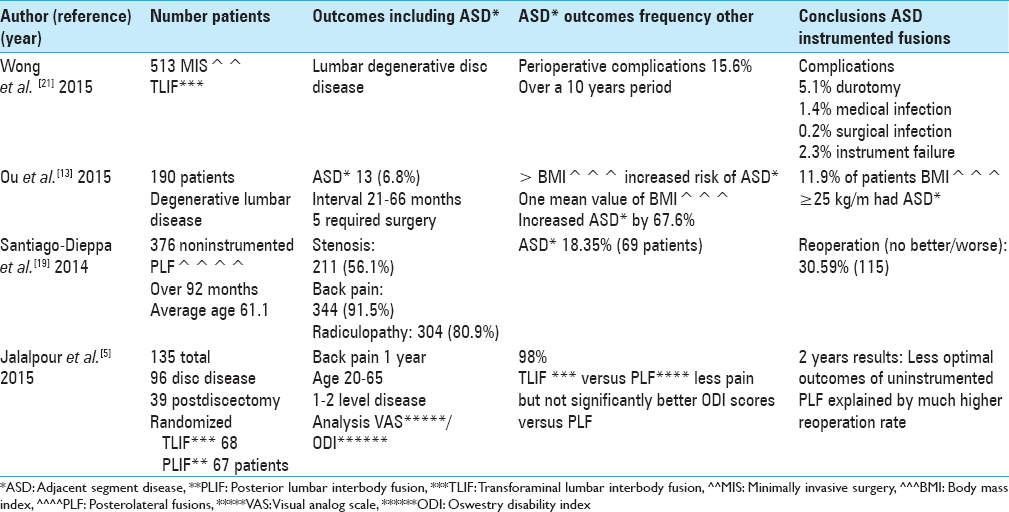
INCIDENCE OF ADJACENT SEGMENT DISEASE FOLLOWING LUMBAR FUSIONS IN 135 CASES OR GREATER [TABLE 1]
Minimally invasive lumbar instrumented fusions: posterior lumbar interbody fusions and transforaminal lumbar interbody fusions: Impact of body mass index (BMI) on adjacent segment disease
In 2015, Ou et al. assessed whether BMI contributed to ASD following 190 lumbar fusions for degenerative disease [
Intraoperative and perioperative complications in minimally invasive transforaminal lumbar interbody fusion
Wong et al. reviewed intraoperative and perioperative complications for 513 consecutively performed MIS TLIF (cohort series) performed for lumbar degenerative disc disease over a 10-year interval [
Long-term outcomes after noninstrumented lumbar arthrodesis
Santiago-Dieppa et al. looked at the frequency of ASD following noninstrumented lumbar fusions performed at one institution for degenerative disease over a 20-year period (median 92 months) [
Randomized controlled trial comparing instrumented transforaminal lumbar interbody fusions versus noninstrumented posterolateral lumbar fusion for the treatment of degenerative lumbar spine disease
Jalalpour et al. compared the 2-year clinical outcomes of TLIF (68 patients: pedicle titanium screw fixation and a porous tantalum interbody spacer with interbody and posterolateral autograft) versus noninstrumented PLF (67 patients; used autograft) procedures for managing chronic low back pain in 135 patients with degenerative disc disease (n = 96) or postdiscectomy syndrome (n = 39) [
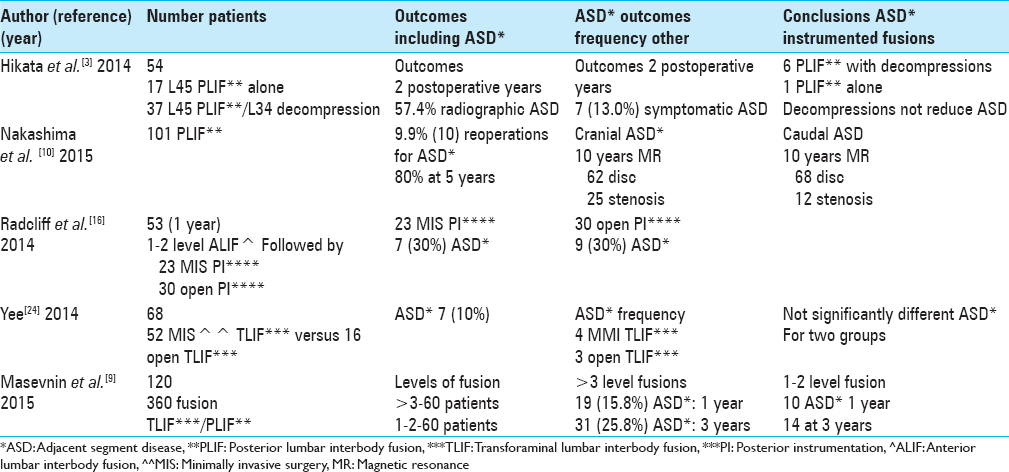
INCIDENCE OF ADJACENT SEGMENT DISEASE FOLLOWING LUMBAR SPINE INSTRUMENTATION IN UP TO 101 CASES [TABLE 2]
Efficacy of posterior lumbar interbody fusion plus cephalad decompression for symptomatic adjacent segment disease
Hikata et al. noted that for 54 patients undergoing L4–L5 PLIF (performed for degenerative spondylolisthesis [DS]), fusion would potentially increase mechanical stress and degenerative changes at the adjacent L3–L4 level [
Risk factors for adjacent segment disease after lumbar fusion
Masevnin et al. studied the frequency of ASD in 120 patients who had 360° lumbar fusions (2007–2012) [
Adjacent segment disease after posterior lumbar interbody fusion with 10 years of follow-up
In 2015, Nakashima et al. followed 101 patients undergoing PLIF who were followed for a minimum of 10 years; they utilized X-ray and MR studies to evaluate ASD at 2, 5, and 10 years postoperatively [
Rate of adjacent segment disease after percutaneous minimally invasive surgery versus open fusion
When Radcliff et al. looked at the frequency of ASD following one or two level anterior lumbar interbody fusions (ALIF) followed by posterior open versus posterior MI lumbar instrumentation, they found that both types of posterior fusions contributed to a 30% incidence of ASD [
Comparison of adjacent segment disease after minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion
In a retrospective cohort study of 68 patients, Yee et al. (2014) evaluated the frequency of ASD following MI TLIF (52 patients) versus open TLIF (16 patients; slightly older); all patients were followed for a minimum of 6 months looking for the onset of symptomatic ASD [
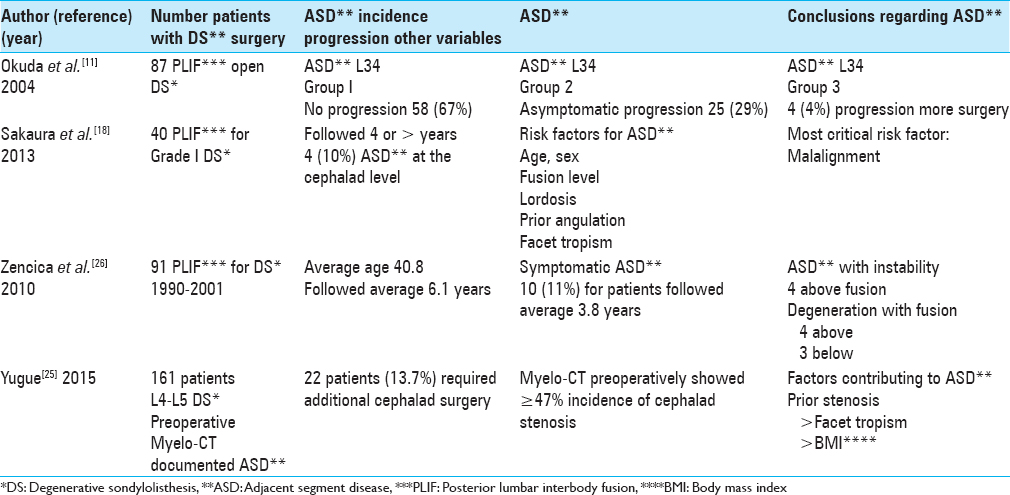
DEGENERATIVE SPONDYLOLISTHESIS: CONCLUSIONS REGARDING ADJACENT SEGMENT DISEASE AND OTHER FACTORS [TABLE 3]
Risk factors for adjacent segment disease after posterior lumbar interbody fusion for degenerative spondylolisthesis
Over a 2-year period, Okuda et al. evaluated the frequency of ASD for 87 patients undergoing PLIF at the L4–L5 level for DS [
Symptomatic adjacent segment disease after posterior lumbar interbody fusion for adult low-grade isthmic spondylolisthesis
Over at least a 4-year period, Sakaura et al. found the frequency of symptomatic ASD to be 10% (4 patients) following 40 PLIF (with pedicle screws) performed for adult low-grade isthmic spondylolisthesis (IS) [
Adjacent segment disease after lumbosacral fusion for spondylolisthesis
In 2010, Zencica et al. evaluated the frequency of ASD (retrospective, X-ray study) following 91 PLIF (transpedicular fixation) performed in patients with spondylolisthesis (e.g., isthmic [70 patients], degenerative [14 patients], or dysplastic [7 patients] spondylolisthesis at the L4–L5 or L5S1 levels); patients were followed an average of 6.1 years [
Cephalad stenosis requiring more surgery following L4–L5 fusion for degenerative spondylolisthesis
Yugue et al. (2015) looked at the frequency and predisposing elements contributing to ASD following 161 single level fusions where patients’ preoperative Myelo-CT studies showed preexisting cephalad stenosis [
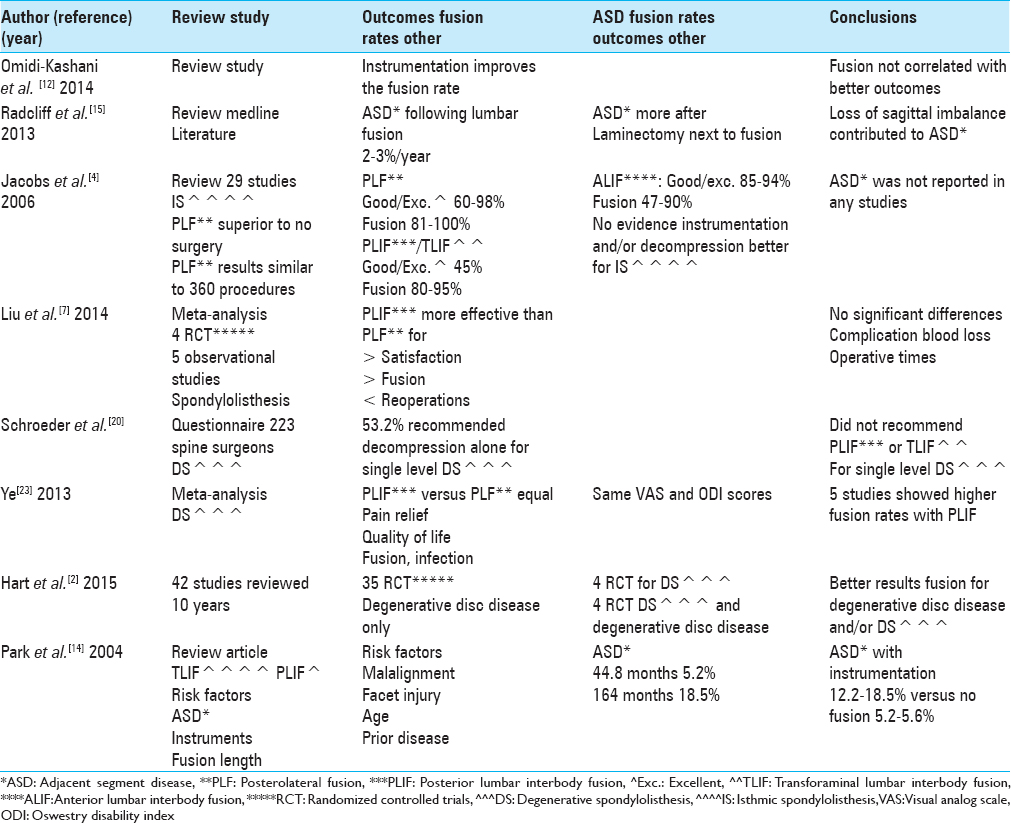
REVIEW ARTICLES ON ADJACENT SEGMENT DISEASE FOLLOWING FUSIONS AND OTHER FACTORS [TABLE 4]
Adjacent segment disease after lumbar or lumbosacral instrumented fusion
In 2004, Park et al. evaluated the frequency of pathological changes (e.g., motion, stenosis, and disc degeneration) adjacent to the levels of prior instrumented lumbar fusions [
Lumbar spinal stenosis: who should be fused?
In 2014, Omidi-Kashani et al., when evaluating lumbar spinal stenosis, asked who should be fused? For patients with radiculopathy but without instability, decompression alone should suffice [
Adjacent segment disease in the lumbar spine following instrumented fusion
Radcliff et al. reviewed the literature in MEDLINE regarding the frequency of ASD adjacent to a prior lumbar fusion [
Fusion for low-grade adult isthmic spondylolisthesis
In 2006, Jacobs et al. evaluated which fusion method resulted in better outcomes (clinical/radiographic) for patients with low-grade IS [
Meta-analysis comparing posterior lumbar interbody fusion versus posterolateral lumbar fusion using transpedicular screw fixation for isthmic spondylolithesis
Ye et al. (2013) performed a comparison between PLIF versus PLF for managing IS, looking at pain relief, quality of life, fusion, and infection rates [
Evaluation of lumbar fusion over the past 10 years: A systematic review
In 2015, Hart et al. looked at 42 RCTs assessing the quality of evidence for lumbar fusions addressing degenerative disc disease (35 RCTs), DS (4 RCTs), and 3 RCTs with both of the former over the last 10 years [
Rationale for the surgical treatment of lumbar degenerative spondylolisthesis
Schroeder et al. evaluated the management of lumbar DS, by looking at multiple variables: age, dynamic instability, and outcomes related to surgeon-related factors [
LUMBAR INTERBODY FUSION VERSUS POSTEROLATERAL LUMBAR FUSION FOR LUMBAR SPONDYLOLISTHESIS; META-ANALYSIS
In 2014, in a meta-analysis of the literature (four randomized controlled and five comparative observational studies), Liu et al. compared the efficacy of PLIF versus PLF for managing lumbar spondylolisthesis [
ADJACENT SEGMENT DISEASE LITERATURE REVIEW OF INSTRUMENTED CERVICAL FUSIONS
In 2014, Saavedra-Pozo et al. reviewed the PubMed literature regarding ASD looking at different cervical spine fusion strategies (e.g., instrumented, dynamic, etc.).[
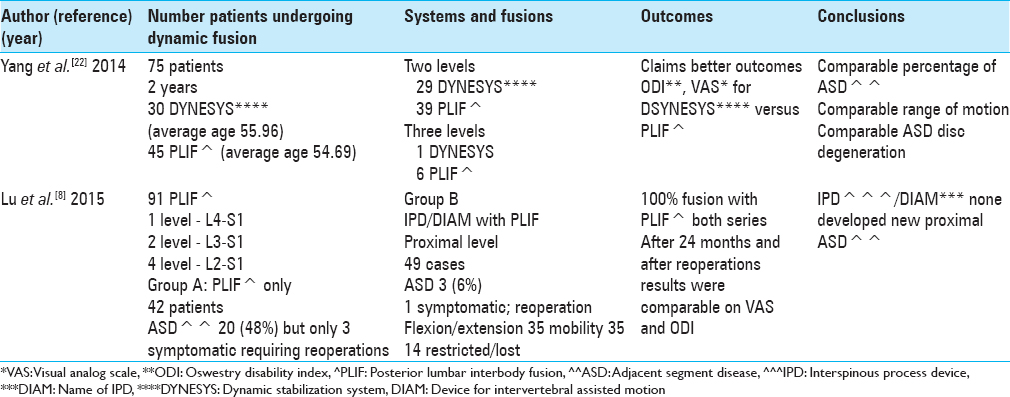
DYNAMIC MODES OF STABILIZATION ADDRESSING ADJACENT SEGMENT DISEASE: DYNAMIC DEVICES RESULT IN COMPARABLE OUTCOMES [TABLE 5]
Short-term outcome of posterior lumbar dynamic stabilization with dynamic stabilization system
In 2014, Yang et al. noted that laminectomy accompanied by lumbar instrumented fusion for degenerative disease too frequently correlated with complications that included; “donor site pain, pseudoarthrosis, nonunion, screw loosening, instrumentation failure, infection, ASD, and degeneration” [
Reduction in adjacent segment disease after multilevel posterior lumbar interbody fusion with/without addition of interbody fusion device proximal to posterior lumbar interbody fusion
In 2015, Lu et al. observed that multilevel lumbar fusions increased the risk of developing ASD [
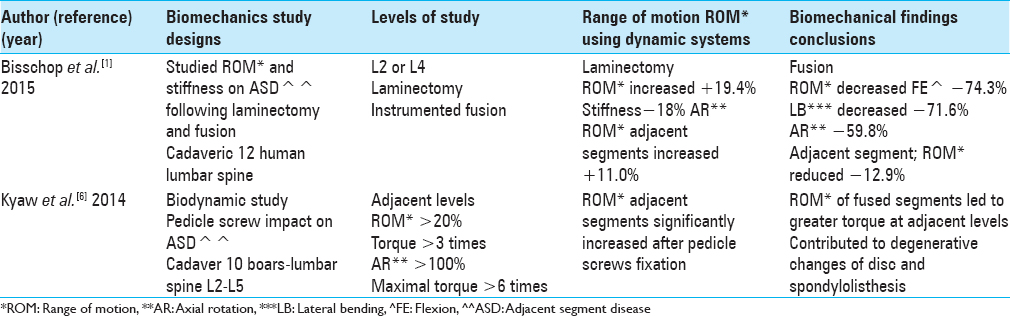
BIODYNAMICS OF ADJACENT SPINAL DISEASE [TABLE 6]
Biodynamics of single-level instrumented lumbar laminectomy on adjacent segment disease biomechanics
In 2015, Bisschop et al. studied the ROM and stiffness of adjacent lumbar spinal segments along with ASD following laminectomy and fusion. Utilizing 12 human lumbar cadaveric spines, a laminectomy at L2 or at L4 was followed by posterior instrumentation.[
BIOMECHANICAL IMPACT OF PEDICLE SCREWS ON ADJACENT SEGMENT DISEASE
In 2014, Kyaw et al. utilizing 10 cadaveric boars spines at the L2–L5 levels, evaluated the biomechanical impact of pedicle screws on ASD in the lumbar spine.[
CONCLUSIONS
This review raises the issue of whether ASD could be substantially reduced by performing fewer lumbar instrumented fusions. ASD occurred in up to 30% of lumbar instrumented fusions whether performed open or minimally invasively, with or without DS accompanying stenosis.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bisschop A, Holewijn RM, Kingma I, Stadhouder A, Vergroesen PP, van der Veen AJ. The effects of single-level instrumented lumbar laminectomy on adjacent spinal biomechanics. Global Spine J. 2015. 5: 39-48
2. Hart R, Hermsmeyer JT, Sethi RK, Norvell DC. Quality and quantity of published studies evaluating lumbar fusion during the past 10 years: A systematic review. Global Spine J. 2015. 5: 207-18
3. Hikata T, Kamata M, Furukawa M. Risk factors for adjacent segment disease after posterior lumbar interbody fusion and efficacy of simultaneous decompression surgery for symptomatic adjacent segment disease. J Spinal Disord Tech. 2014. 27: 70-5
4. Jacobs WC, Vreeling A, De Kleuver M. Fusion for low-grade adult isthmic spondylolisthesis: A systematic review of the literature. Eur Spine J. 2006. 15: 391-402
5. Jalalpour K, Neumann P, Johansson C, Hedlund R. A randomized controlled trial comparing transforaminal lumbar interbody fusion and uninstrumented posterolateral fusion in the degenerative lumbar spine. Global Spine J. 2015. 5: 322-8
6. Kyaw TA, Wang Z, Sakakibara T, Yoshikawa T, Inaba T, Kasai Y. Biomechanical effects of pedicle screw fixation on adjacent segments. Eur J Orthop Surg Traumatol. 2014. 24: S283-7
7. Liu X, Wang Y, Qiu G, Weng X, Yu B. A systematic review with meta-analysis of posterior interbody fusion versus posterolateral fusion in lumbar spondylolisthesis. Eur Spine J. 2014. 23: 43-56
8. Lu K, Liliang PC, Wang HK, Liang CL, Chen JS, Chen TB. Reduction in adjacent-segment degeneration after multilevel posterior lumbar interbody fusion with proximal DIAM implantation. J Neurosurg Spine. 2015. 23: 190-6
9. Masevnin S, Ptashnikov D, Michaylov D, Meng H, Smekalenkov O, Zaborovskii N. Risk factors for adjacent segment disease development after lumbar fusion. Asian Spine J. 2015. 9: 239-44
10. Nakashima H, Kawakami N, Tsuji T, Ohara T, Suzuki Y, Saito T. Adjacent segment disease after posterior lumbar interbody fusion: Based on cases with a minimum of 10 years of follow-up. Spine (Phila Pa 1976). 2015. 40: E831-41
11. Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976). 2004. 29: 1535-40
12. Omidi-Kashani F, Hasankhani EG, Ashjazadeh A. Lumbar spinal stenosis: Who should be fused. An updated review?. Asian Spine J. 2014. 8: 521-30
13. Ou CY, Lee TC, Lee TH, Huang YH. Impact of body mass index on adjacent segment disease after lumbar fusion for degenerative spine disease. Neurosurgery. 2015. 76: 396-401
14. Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine (Phila Pa 1976). 2004. 29: 1938-44
15. Radcliff KE, Kepler CK, Jakoi A, Sidhu GS, Rihn J, Vaccaro AR. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013. 13: 1339-49
16. Radcliff KE, Kepler CK, Maaieh M, Anderson DG, Rihn J, Albert T. What is the rate of lumbar adjacent segment disease after percutaneous versus open fusion?. Orthop Surg. 2014. 6: 118-20
17. Saavedra-Pozo FM, Deusdara RA, Benzel EC. Adjacent segment disease perspective and review of the literature. Ochsner J. 2014. 14: 78-83
18. Sakaura H, Yamashita T, Miwa T, Ohzono K, Ohwada T. Symptomatic adjacent segment pathology after posterior lumbar interbody fusion for adult low-grade isthmic spondylolisthesis. Global Spine J. 2013. 3: 219-24
19. Santiago-Dieppa D, Bydon M, Xu R, De la Garza-Ramos R, Henry R, Sciubba DM. Long-term outcomes after non-instrumented lumbar arthrodesis. J Clin Neurosci. 2014. 21: 1393-7
20. Schroeder GD, Kepler CK, Kurd MF, Vaccaro AR, Hsu WK, Patel AA. Rationale for the surgical treatment of lumbar degenerative spondylolisthesis. Spine (Phila Pa 1976). 2015. p.
21. Wong AP, Smith ZA, Nixon AT, Lawton CD, Dahdaleh NS, Wong RH. Intraoperative and perioperative complications in minimally invasive transforaminal lumbar interbody fusion: A review of 513 patients. J Neurosurg Spine. 2015. 22: 487-95
22. Yang M, Li C, Chen Z, Bai Y, Li M. Short term outcome of posterior dynamic stabilization system in degenerative lumbar diseases. Indian J Orthop. 2014. 48: 574-81
23. Ye YP, Xu H, Chen D. Comparison between posterior lumbar interbody fusion and posterolateral fusion with transpedicular screw fixation for isthmic spondylolithesis: A meta-analysis. Arch Orthop Trauma Surg. 2013. 133: 1649-55
24. Yee TJ, Terman SW, La Marca F, Park P. Comparison of adjacent segment disease after minimally invasive or open transforaminal lumbar interbody fusion. J Clin Neurosci. 2014. 21: 1796-801
25. Yugué I, Okada S, Masuda M, Ueta T, Maeda T, Shiba K. Risk factors for adjacent segment pathology requiring additional surgery after single-level spinal fusion: Impact of pre-existing spinal stenosis demonstrated by preoperative myelography. Eur Spine J. 2015. p.
26. Zencica P, Chaloupka R, Hladíková J, Krbec M. Adjacent segment degeneration after lumbosacral fusion in spondylolisthesis: A retrospective radiological and clinical analysis. Acta Chir Orthop Traumatol Cech. 2010. 77: 124-30