- Department of Neurology, Loma Linda University Medical Center, Loma Linda, California, United States
- Advanced Oncology, West Covina, California, United States
- Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States
- School of Medicine, Loma Linda University, Loma Linda, California, United States.
Correspondence Address:
Warren W. Boling, Department of Neurosurgery, Loma Linda University Medical Center, Loma Linda, California, United States.
DOI:10.25259/SNI_485_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Taylor Anne Wilson1, Joseph I. Kang Jr2, Lei Huang3, Alexandra Vacaru4, Kevin Nogueira Martins4, Warren W. Boling3. Adjuvant proton beam therapy in patients with grade 2 meningiomas. 01-Mar-2024;15:62
How to cite this URL: Taylor Anne Wilson1, Joseph I. Kang Jr2, Lei Huang3, Alexandra Vacaru4, Kevin Nogueira Martins4, Warren W. Boling3. Adjuvant proton beam therapy in patients with grade 2 meningiomas. 01-Mar-2024;15:62. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12782
Abstract
Background: The World Health Organization (WHO) grade 2 meningiomas behave aggressively with a high proclivity toward recurrence despite maximal surgical resection. Our institution, a pioneer of proton therapy, uses exclusively proton beam radiation, and thus, we present a retrospective cohort analysis of patients with WHO grade 2 meningiomas treated with adjuvant proton beam therapy (PBT) at our institution between 2007 and 2019. The effects of adjuvant PBT were evaluated.
Methods: Data collected include diagnosis, gender, histological subtype, WHO grade, the extent of surgical resection, adjuvant PBT radiation, details of the PBT radiation, recurrence, any additional PBT radiation, systemic medical therapy, and disease-specific survival.
Results: Among the WHO grade 2 meningiomas (n = 50) recommended PBT, 80% and 78% of patients with gross-total resection (GTR) and subtotal resection (STR), respectively, followed through with PBT. The median radiation dose of PBT was 59.5 Gy and 59.92 Gy for patients with GTR and STR, respectively, with a median of 33 fractions delivered in 1.8 Gy doses for both groups. Combined 3-year progression-free survival (PFS) was 96%, and 5-year PFS was 92%. Combined overall survival was 95% at five years. Minimal radiation side effects were reported with no grade 3 or higher toxicities.
Conclusion: Our results suggest that adjuvant PBT is well tolerated with minimal radiation toxicity. Alternative to photon radiation, PBT may be considered at least as safe and effective for adjuvant treatment of WHO grade 2 meningiomas when it is available.
Keywords: Anaplastic meningioma, Atypical meningioma, Meningioma, Proton beam therapy, Radiation therapy
INTRODUCTION
Despite maximal surgical and medical treatment, the World Health Organization (WHO) grade 2 and grade 3 meningiomas behave aggressively with a high proclivity toward recurrence. The standard of care for partially resected WHO grade 2 and all WHO grade 3 meningiomas is surgical resection followed by adjuvant radiation. The role of adjuvant radiation for completely resected WHO grade 2 meningiomas remains debated. Regardless of treatment, these meningiomas often recur, presenting with more aggressive, higher-grade features with increasing resistance to current treatment modalities.[
Due to advancements in radiation techniques, several new options have emerged for the delivery of radiation to meningiomas. While photons are utilized to deliver radiation conventionally, particle therapy, using protons or carbon ions, is a newer modality to deliver radiation. Similar to stereotactic radiation therapy with photons, particle therapy also utilizes the stereotactic method. Compared with photons, protons, and carbon ions more precisely deliver higher radiation doses to tumor cells while limiting radiation to adjacent brain structures.[
Our institution, a pioneer of proton therapy treatment, uses exclusively proton beam radiation, and thus, we present a retrospective analysis of a cohort of appropriate patients with WHO grade 2 meningiomas who were treated with adjuvant proton beam therapy (PBT).
MATERIALS AND METHODS
This study was approved by the Loma Linda University Institutional Review Board (IRB No. 5190476). Patients treated for a WHO grade 2 meningioma at our institution between 2007 and 2019 were included in the study. Due to the limited treatment of patients with the WHO grade 3 meningioma, these patients were excluded from the study analysis. In 2007, the WHO grading system was updated to include brain invasion as a criterion for classification as grade 2, and thus, tumor grading from 2007 forward should be more homogeneous than with years prior. The 2007 time point to ensure similar diagnostic criteria and subsequent management decisions was applied to all patients in the study. Patients diagnosed at our institution but treated elsewhere were excluded from the study. Patients who were treated at another institution and established care for follow-up after treatment were also excluded from the study.
All patients were treated at Loma Linda University Medical Center’s hospital-based proton therapy facility. They were immobilized with thermoplastic masks and received treatment planning using 3D contrast-enhanced computed tomography with magnetic resonance imaging (MRI) registration. The clinical target volume included a 5 mm expansion, and proton beam-specific apertures and compensators were designed using proton treatment planning software. Most patients received 2–4 fields of passively scattered proton beams, with a 2–3 mm planning target volume margin added to account for penumbra and immobilization uncertainties. The dose equivalent to photons was calculated using a relative biological effectiveness value of 1.1. The general policy was to treat the entire tumor bed and any residual enhancing disease to 59.4 GyE in 33 fractions, but alternative fractionation or dose escalation was at the physician’s discretion. Organs at risk were limited to standard constraints, including a Dmax of 54Gy for optics and 60Gy for the brainstem. Data collected include diagnosis, gender, histological subtype, WHO grade, extent of surgical resection, adjuvant radiation and details of the radiation, recurrence, any additional radiation, chemotherapy, or other systemic medical therapy, and disease-specific survival. Adverse events from radiation were also evaluated. All patients treated with adjuvant radiation at our institution received PBT.
Data were analyzed using SPSS version 26.0 (IBM SPSS Statistics for MacIntosh. Armonk, NY: IBM Corp, 2019). The Kolmogorov–Smirnov test for normality determined data is not normally distributed, so non-parametric statistics were used when appropriate.
RESULTS
WHO grade 2 meningiomas
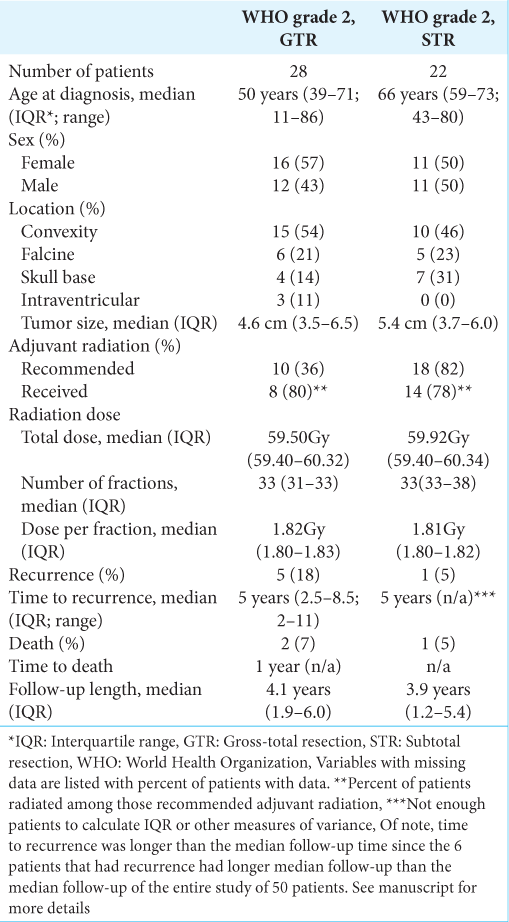
Fifty patients had WHO grade 2 meningiomas with 54% females. The median age of diagnosis was 62 years, ranging from 11 to 86 years. More detailed demographic, tumor, and treatment information is provided in
Convexity meningiomas were the most common (50%), followed by falcine (22%) and anterior skull base (22%) meningiomas. Three patients had intraventricular meningiomas. Tumor size varied from the smallest meningioma, measuring 1.8 cm, to the largest, measuring 10.1 cm, with a median of 5.0 cm.
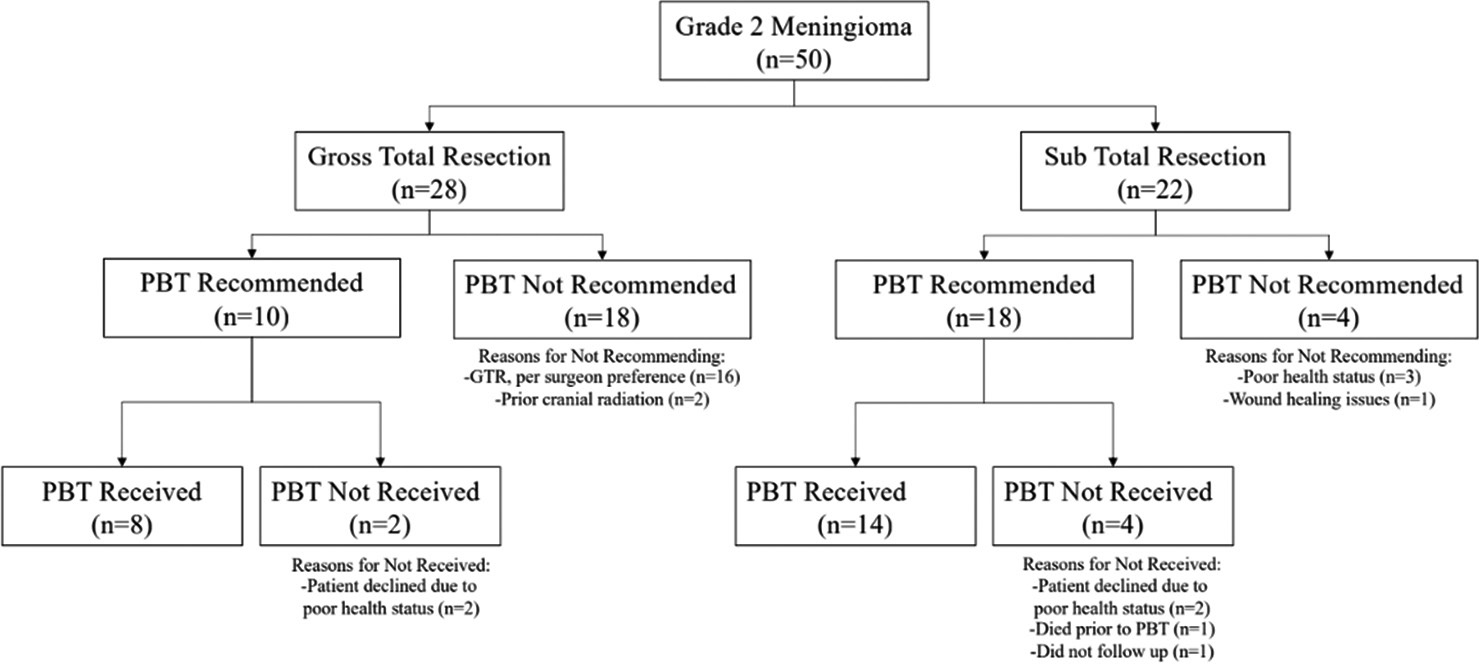
On histopathology, the WHO grade 2 meningiomas were 90% atypical, 4% choroid, 4% clear cell, and 2% fibrous subtypes. Of note, the fibrous subtype had evidence of brain invasion, which independently classifies it as WHO grade 2. Gross total resection (GTR) was achieved in 22 patients (44%) and subtotal resection (STR) in 28 patients (56%).
Adjuvant radiation using PBT was recommended in 36% of patients with GTR and 82% of STR as initial treatment. Adjuvant radiation was recommended at physician discretion based on patient performance status, extent of tumor resection, initial tumor size, extent of tumor invasion of local structures, prior infield radiation treatment, and tumor proliferation index [
Throughout the study, among all patients with WHO grade 2 meningiomas, there were six patients with recurrences with a 3-year progression-free survival (PFS) of 96% and a 5-year PFS of 92%. Among those patients with GTR, progression occurred in 5 patients (18%) and one patient (5%) with STR. Among those with GTR, the 3-year PFS was 93%, and the 5-year PFS was 89%. Among those with STR, the 3-year PFS was 100%, and the 5-year PFS was 95%. During initial treatment, adjuvant radiation of PBT was previously recommended for all six patients who ultimately recurred, yet only 50% were radiated. All recurrent meningiomas remained WHO grade 2 with no change in histopathologic subtype and no gross evidence of malignant progression such as a new brain invasion.
Among all WHO grade 2 meningiomas, three total patients died for an overall survival (OS) rate of 94% at 3 and 5 years. There was 93% (26 of 28 patients) and 95% (21 of 22 patients) OS at 3 and 5 years in those with GTR and STR, respectively. One patient with STR died from meningitis shortly after surgery before receiving radiation recommendations. One patient with GTR was recommended radiation, but he ultimately declined the treatment. The other patient with GTR was not recommended for adjuvant radiation. Both GTR patients died within a year, one of cardiac arrest and the other a stroke. None of the deceased patients received adjuvant radiation with PBT before death. Furthermore, the three deceased patients all died during initial treatment after meningioma diagnosis, not from recurrence or recurrence-related complications.
There was minimal severity of acute and long-term radiation adverse effects with no grade 3 or higher toxicities. Among the 36% of patients who reported minor symptoms, the most common acute effects included headache (16%), memory changes (9%), fatigue (6%), and the most common long-term effects included speech changes (4%), hearing loss (2%), and hair loss (2%).
DISCUSSION
Adjuvant radiation is considered the standard of care for patients with WHO grade 3 and incompletely resected WHO grade 2 meningiomas. The role of adjuvant radiation for patients with completely resected WHO grade 2 meningiomas remain debated. Regardless of treatment, the WHO grade 2 and 3 meningiomas have a penchant for recurrence. Recurrent meningiomas often exhibit higher-grade features, becoming refractory to standard surgical and radiation therapies.
Conventional radiation utilizes photons to deliver radiation as fractionated radiotherapy or SRS techniques. Evidence in the literature supports photon radiation as a generally safe, effective, and widely available treatment for appropriate patients with WHO grade 2 and 3 meningiomas. The benefit of SRS remains to be clearly defined in the literature. Due to the propensity of high-grade meningiomas to infiltrate the dura and other adjacent structures combined with the smaller margins used for SRS, the 5-year PFS is relatively low, ranging from 34% to 56% with recurrences often occurring elsewhere in untreated areas of resection cavities.[
RTOG 0539 and EORTC 22042-26041 are two prospective non-randomized trials that have compared fractionated radiotherapy to historical control for the treatment of high-grade meningiomas.[
A major disadvantage to photon radiation is off-target radiation damage to the surrounding healthy tissue. Particularly in recurrent meningiomas, photon radiation utility is often limited by the surrounding healthy tissue’s tolerance to more radiation.[
Although there is a paucity of high-quality evidence on particle therapy in the treatment of WHO grade 2 and 3 meningiomas, several smaller retrospective studies have been published.[
However, few studies evaluate solely PBT for the treatment of WHO grade 2 meningiomas, and to the best of our knowledge, the present study is the largest study with 50 patients. Furthermore, ours is the only study that delineates between extent of resection and does not combine results for initial and recurrent treatments. Fortunately for our patients, only a minority of patients suffered recurrence or mortality; however, this small number of patients limited the extent of subgroup statistical analysis on those patients. Based on the results of the present study, PBT appears to be at least as effective as photon based fractionated radiotherapy for adjuvant treatment of the WHO grade 2 meningiomas. As described above, the RTOG 0539 and EORTC 22042-26041 reported 3-year PFS of 98.3% and 88.7%, respectively. The present study found a 3-year PFS of 96% among all patients treated with PBT. Furthermore, PBT also appears to be at least as safe as SRS, with the above studies reporting 0% and 14.3% of patients with grade 3 or higher radiation toxicity, and the present study found no grade 3 or higher radiation toxicities.
Limitations include the small sample size and short length of follow-up. Another limitation includes analysis of the patients who died. Of the patients with grade 2 meningioma, three patients died, making subgroup analysis of these patients challenging. Interestingly, none of these patients died of complications related to meningioma. Finally, another limitation of our study is the difficulty with comparing photon radiation with PBT as our institution does not have photon therapy for comparison, and according to the literature, many of the studies on photon-based therapies for the treatment of meningioma are small, poorly powered studies with inconsistent results.[
CONCLUSION
PBT is considered at least as safe and effective as conventional photon radiation for both initial treatment and re-irradiation of recurrent meningiomas. The results of this study suggest that adjuvant PBT is well tolerated with minimal radiation side effects and no grade 3 or higher radiation toxicity reported. PBT is associated with improvements in the progression, recurrence-free survival, and OS in appropriate patients with WHO grade 2 meningiomas compared with historical data, and it appears to be at least as effective and safe as SRS. Alternative to photon radiation, PBT may be considered safe and effective for adjuvant treatment of WHO grade 2 meningiomas when it is available.
Ethical approval
This study was approved by the Loma Linda University Institutional Review Board (IRB No. 5190476) 03 February 2020.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adeberg S, Harrabi SB, Verma V, Bernhardt D, Grau N, Debus J. Treatment of meningioma and glioma with protons and carbon ions. Radiat Oncol. 2017. 12: 193
2. Coggins WS, Pham NK, Nguyen AV, Branch DW, Guillet JY, Korst G. A systematic review of ion radiotherapy in maintaining local control regarding atypical and anaplastic meningiomas. World Neurosurg. 2019. 132: 282-91
3. Combs SE, Hartmann C, Nikoghosyan A, Jäkel O, Karger CP, Haberer T. Carbon ion radiation therapy for high-risk meningiomas. Radiother Oncol. 2010. 95: 54-9
4. El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S. Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiat Oncol. 2018. 13: 86
5. Kosaki K, Ecker S, Habermehl D, Rieken S, Jäkel O, Herfarth K. Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat Oncol. 2012. 7: 44
6. Madani I, Lomax AJ, Albertini F, Trnková P, Weber DC. Dose-painting intensity-modulated proton therapy for intermediate-and high-risk meningioma. Radiat Oncol. 2015. 10: 72
7. McDonald MW, Plankenhorn DA, McMullen KP, Henderson MA, Dropcho EJ, Shah MV. Proton therapy for atypical meningiomas. J Neurooncol. 2015. 123: 123-8
8. Mozes P, Dittmar JO, Habermehl D, Tonndorf-Martini E, Hideghety K, Dittmar A. Volumetric response of intracranial meningioma after photon or particle irradiation. Acta Oncol. 2017. 56: 431-7
9. Murray FR, Snider JW, Bolsi A, Lomax AJ, Walser M, Kliebsch U. Long-term clinical outcomes of pencil beam scanning proton therapy for benign and non-benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2017. 99: 1190-8
10. Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of World Health Organization grade II and III intracranial meningiomas: Treatment results on the basis of a 22-year experience. Cancer. 2012. 118: 1048-54
11. Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy. Semin Oncol. 2019. 46: 226-32
12. Rogers L, Zhang P, Vogelbaum MA, Perry A, Ashby LS, Modi JM. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018. 129: 35-47
13. Sun SQ, Cai C, Murphy RK, DeWees T, Dacey RG, Grubb RL. Radiation therapy for residual or recurrent atypical meningioma: The effects of modality, timing, and tumor pathology on long-term outcomes. Neurosurgery. 2016. 79: 23-32
14. Weber DC, Ares C, Villa S, Peerdeman SM, Renard L, Baumert BG. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother Oncol. 2018. 128: 260-5
15. Weber DC, Bizzocchi N, Bolsi A, Jenkinson MD. Proton therapy for intracranial meningioma for the treatment of primary/recurrent disease including re-irradiation. Front Oncol. 2020. 10: 558845
16. Weber DC, Grau C, Lim PS, Georg D, Lievens Y. Bringing Europe together in building clinical evidence for proton therapy-the EPTN-ESTRO-EORTC endeavor. Acta Oncol. 2019. 58: 1340-2
17. Williams BJ, Salvetti DJ, Starke RM, Yen CP, Sheehan JP. Stereotactic radiosurgery for WHO II and III meningiomas: Analysis of long-term clinical and radiographic outcomes. J Radiosurg SBRT. 2013. 2: 183-91
18. Wilson TA, Huang L, Ramanathan D, Lopez-Gonzalez M, Pillai P, De Los Reyes K. Review of atypical and anaplastic meningiomas: Classification, molecular biology, and management. Front Oncol. 2020. 10: 565582
19. Zhang M, Ho AL, D’Astous M, Pendharkar AV, Choi CY, Thompson PA. CyberKnife stereotactic radiosurgery for atypical and malignant meningiomas. World Neurosurg. 2016. 91: 574-81.e1