- Department of Neurology and Neurosurgery, Universidade Federal de São paulo, São Paulo, Brazil
- Department of Medicine, Faculty of Medicine, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- Department of Medicine, Max Planck University Center, Indaiatuba, Brazil
- Department of Neurosurgery, Hospital Beneficência Portuguesa de São Paulo, São Paulo - SP, Brazil. Brazil
Correspondence Address:
Feres Chaddad-Neto, Department of Neurology and Neurosurgery, Federal University of São Paulo, Rua Napoleão de Barros, São Paulo, Brazil.
DOI:10.25259/SNI_843_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luis Gustavo Biondi-Soares1, Filipi Fim Andreão2, Luis Ángel Canache Jiménez1, Felipe Salvagni Pereira1, Lucca Biolcati Palavani3, René Alejandro Apaza-Tintaya1, Dmitriy Korotkov1, José Maria de Campos Filho1, Raphael Wuo-Silva1, Feres Chaddad-Neto1,4. Advanced fluorescence techniques in white matter fiber visualization for neurosurgical training. 29-Nov-2024;15:447
How to cite this URL: Luis Gustavo Biondi-Soares1, Filipi Fim Andreão2, Luis Ángel Canache Jiménez1, Felipe Salvagni Pereira1, Lucca Biolcati Palavani3, René Alejandro Apaza-Tintaya1, Dmitriy Korotkov1, José Maria de Campos Filho1, Raphael Wuo-Silva1, Feres Chaddad-Neto1,4. Advanced fluorescence techniques in white matter fiber visualization for neurosurgical training. 29-Nov-2024;15:447. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13251
Abstract
Background: Neurosurgical training requires a deep understanding of brain anatomy, especially white matter fiber pathways, to enhance surgical precision. Traditional dissection techniques, such as Klingler’s white matter dissection, are essential, but newer methods can provide additional clarity. This study explores the application of a fluorescent-assisted technique to improve the visualization and understanding of white matter fibers during neurosurgical training.
Methods: Twelve human brains were dissected following Klingler’s protocol, with a focus white matter fiber pathway. Fluorescent alcoholic and oily solutions were applied to highlight the fibers. Ultraviolet (UV) blacklight and yellow monochromatic filters were used to enhance visualization. Dissections were documented through photography, and the effectiveness of the fluorescent techniques was analyzed.
Results: The application of UV light and fluorescent solutions enhanced the visualization of fiber pathways, particularly in regions with irregular fibers, such as the sagittal stratum. The alcoholic solution flowed along the anatomical paths of the fibers, aiding in their differentiation. The oily solution proved effective in specific areas, such as the internal capsule. The use of fluorescent techniques allowed for improved identification and anatomical detailing of major white matter tracts.
Conclusion: The fluorescent-assisted dissection technique significantly enhances the visualization of white matter fibers, offering a valuable tool for neurosurgical training. This method deepens anatomical understanding, provides a three-dimensional perspective of brain structures, and can improve surgical outcomes. The study suggests potential applications in other fields, such as glioma simulation and bypass patency testing.
Keywords: Fluorescent technique, Neurosurgery, Neurosurgical training, White matter fibers
INTRODUCTION
Neurosurgery as a discipline requires the administration of considerable time in the laboratory to understand the anatomy in depth and develop microsurgical techniques. This has generated today the need to gather and simulate conditions that are really applicable in surgery.
When we talk about anatomical understanding, we must necessarily focus on discovering in depth the internal structure of the brain, its deep nuclei, and its white fibers. Given this panorama, it is well known that the fiber dissection technique helps in the structural understanding and arrangement of the gray and white matter in an additional three-dimensional aspect. The dissection of the white matter involves carefully isolating and studying the neural pathways to understand the networks that underlie human cognition, behavior, and sensorimotor functions.[
Klingler’s method of white matter dissection has advanced neuroscience significantly.[
This study aims to innovate neurosurgical training by introducing a fluorescent-assisted technique in fiber dissection to highlight white matter fibers’ pathways. This method enhances understanding of brain fiber anatomy and function in cadaveric studies, advancing neurosurgical education and practice.
MATERIALS AND METHODS
Twelve formalin-fixed and frozen human brains (24 hemispheres) were preserved at −16°C for 2 weeks. Dissection followed Klingler’s protocol under a surgical microscope (× 6–× 40 magnification), using wooden spatulas, cotton swabs, and Rhoton microsurgical instruments after removing dura mater, arachnoid, and vascular structures.[
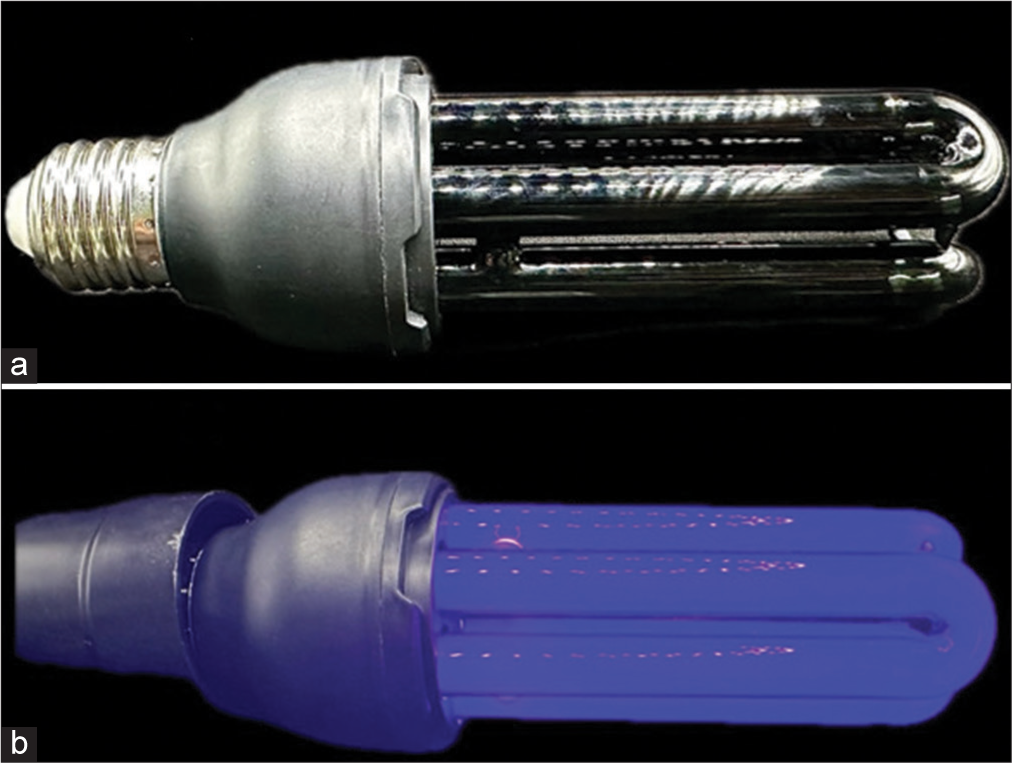
Colored fluorescent solutions (alcoholic and oily) were applied with a cotton swab. The alcoholic solution (VOLTSHOW®, São Paulo, SP, Brazil) was applied following a gravity-guided technique in gravity itself directing the solution along the irregular pathways of the white fibers. This ensures consistent highlighting of the fiber edges, and it can only be observed under ultraviolet (UV) light (with or without a filter). The oily solution (COLORMAKE®, São Paulo, SP, Brazil) is applied in more uniform sections lacking fiber irregularities, where gravity’s assistance in highlighting is inapplicable. Minimal solution was used to avoid anatomical deviation from the fibers pathway [
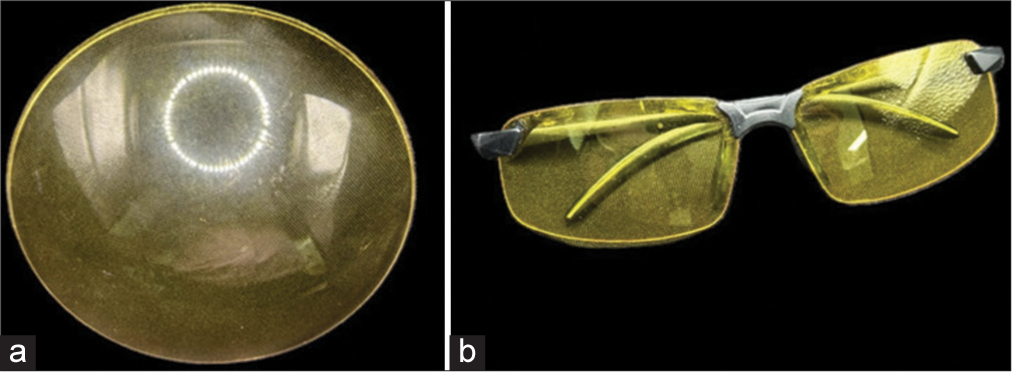
The brain was then frozen for 2 h and observed under a microscope with a yellow monochromatic glass filter and 40-watt neon black UV light. Images were captured and analyzed to document each stage of the process [
RESULTS
Black UV light and yellow monochromatic glass filter
The application of black UV light to the brain specimen was instrumental in accentuating structural peculiarities observed during and after dissection. It highlighted irregularities and, most notably, the depth differences between adjacent structures. To optimize the light spectrum captured by our camera lenses [
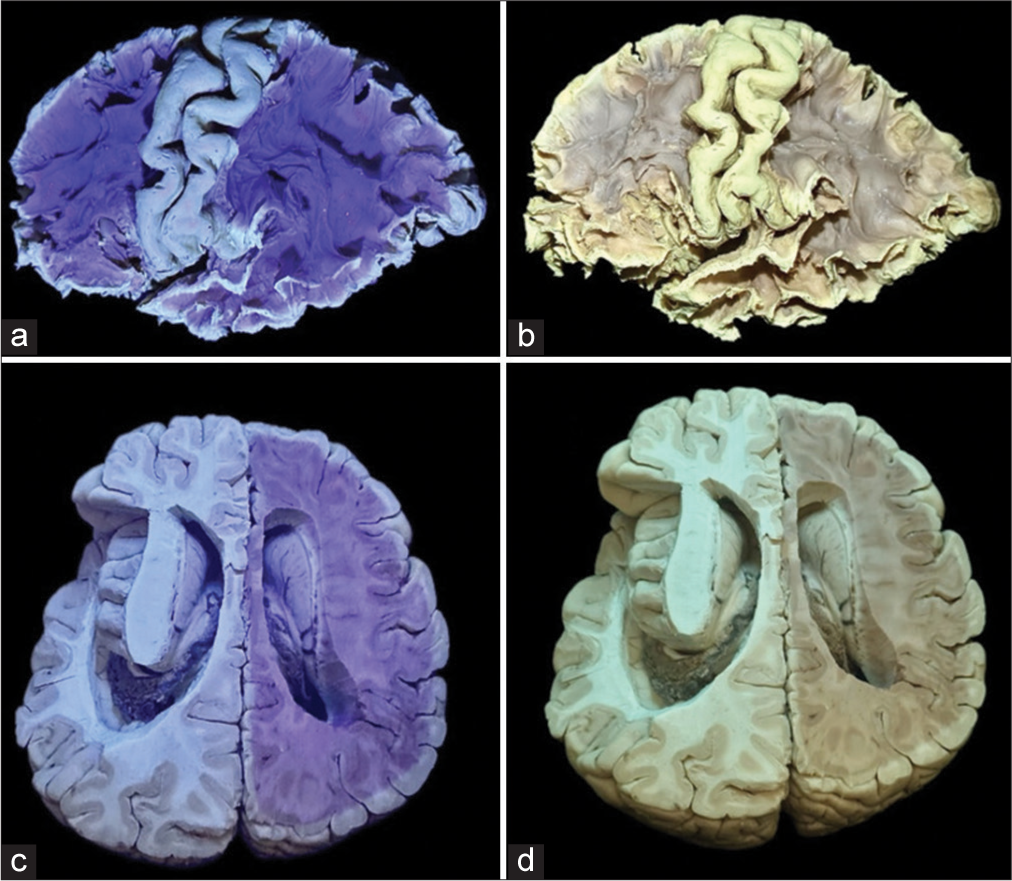
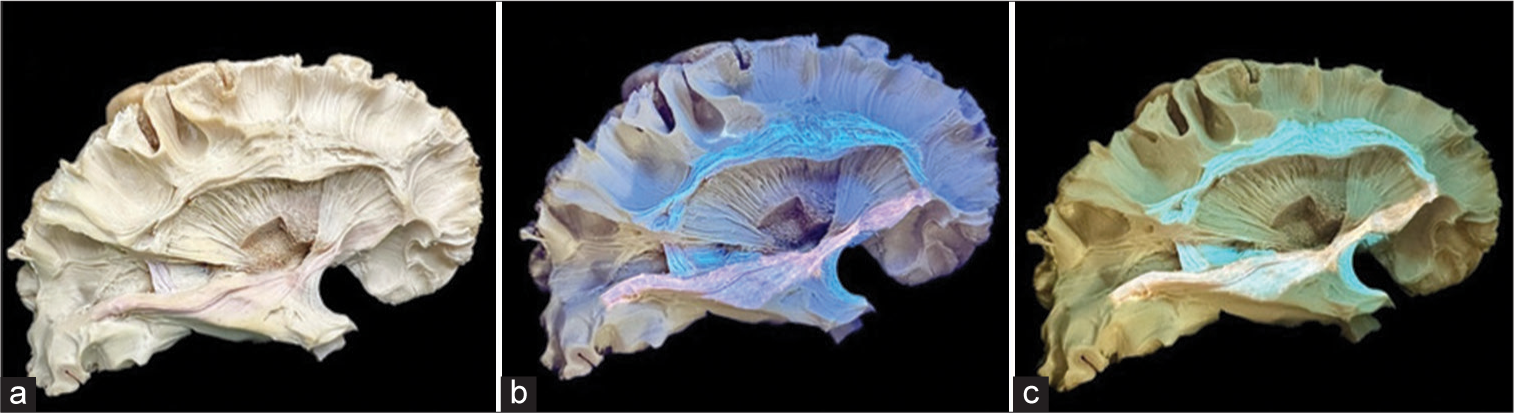
Figure 5:
(a and b) Lateral cerebral surface dissections were performed following the application of black ultraviolet (UV) light, revealing distinctive contrasts in prefilter and postfilter images, respectively. (c and d) Axial cross-sectional analysis of the brain was conducted. The left hemisphere of this specimen underwent dissection in the preceding week, while the right hemisphere was resected during the photographic session. It is noteworthy that the heightened prominence of black UV light contrast correlates with the timing of the specimen dissection, thereby enhancing the efficacy of the anatomical study.
Fluorescent solutions
During neuroanatomical dissections, we observed that the dissected white fibers displayed an irregular surface, which allowed the fluorescent alcoholic solution to flow along their anatomical paths under the influence of gravity. Given the numerous overlapping fibers, particularly in the sagittal stratum, this technique proved useful in visually differentiating these fibers, thereby enhancing their identification during laboratory training. The oily solution did not precisely follow the same route as the alcoholic solution, but it proved valuable in emphasizing specific areas, such as the internal capsule, particularly in axial cross-sections. Through meticulous dissections, we successfully highlighted and anatomically detailed the trajectory of these fibers [
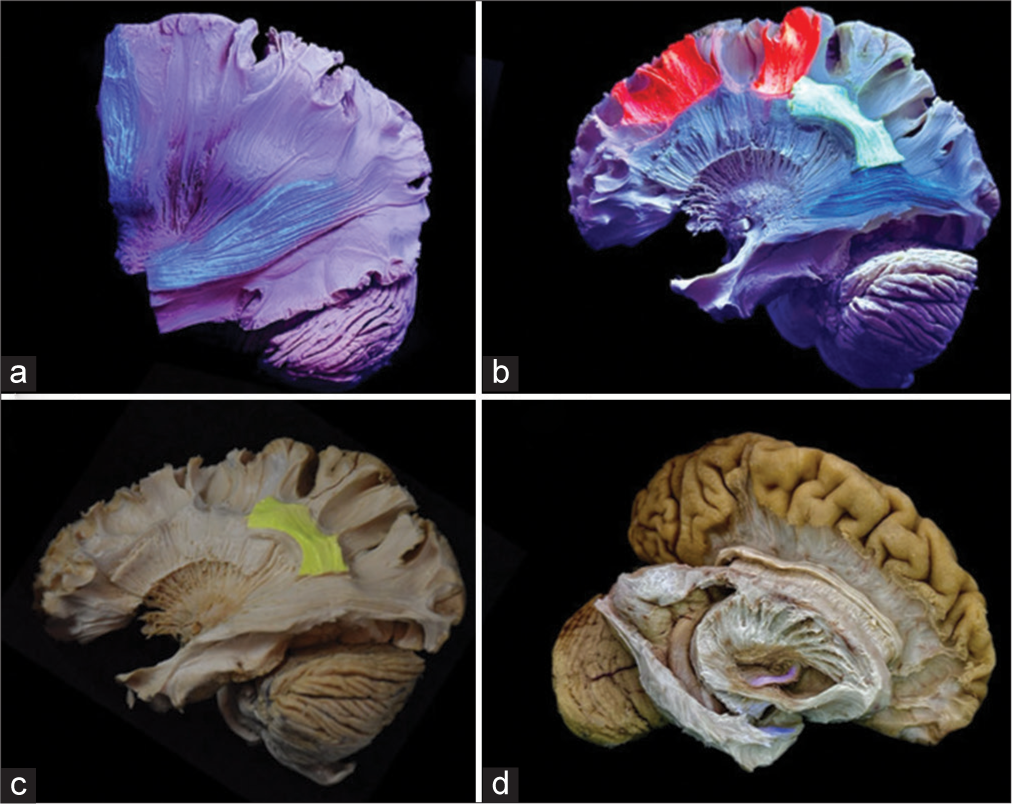
Figure 6:
(a) Hemispheric white matter dissection using the alcoholic fluorescent solution. The lateral surface of the brain reveals fluorescent blue-colored fibers of the stratum sagittale in the antero-posterior orientation and fibers of the corticomedullary tract in the supero-inferior orientation. Notably, the contrast highlights the clear and comprehensible delineation of pathways between the fluorescing fibers and those devoid of fluorescence. (b) Dissected lateral brain surface using oily fluorescent solution unveils white matter fibers, featuring short association u-shaped fibers in fluorescent red, a segment of the arcuate fasciculus in fluorescent green, and a segment of the inferior fronto-occipital fasciculus in fluorescent blue. (c) A specifically delineated segment of the arcuate fasciculus is highlighted in yellow ink. (d) Dissection of the right hemisphere displays the anterior commissure, distinguished by a light red coloration.
Gravity-guided technique
After completing the meticulous white fiber dissection training and identifying cortical landmarks along with their projections to deep structures, select the fiber to be studied. Position the UV light approximately 40 cm from the specimen and adjust the yellow monochromatic lens for the best view. Ensure the specimen is completely dry by letting it sit out of the formaldehyde or alcohol solution for at least 30 min. Tilt the specimen to a 60° angle, aligning it with the dissected fibers.
Next, gently place a cotton swab soaked in the alcoholic solution at the highest point where the fibers begin. Take care not to overflow the solution onto adjacent structures and avoid blowing or making sudden movements. Allow the solution to dry naturally, reapplying as needed until it fully traverses the fiber. Once the procedure is complete, place the specimen in the freezer for 20 min. Finally, proceed with your studies and photography [
This technique is a valuable tool in the study and training of neurosurgeons, as it requires a minimum theoretical understanding of white fibers for effective use. It can be applied during dissection to assist in identifying these fibers or afterward to highlight them.
We highlighted the structural configuration, trajectory, and relationships of the corticospinal tract, superior longitudinal fasciculus, arcuate fasciculus, temporal stem, uncinate fasciculus, inferior fronto-occipital fasciculus, optic radiations [
Figure 7:
Lateral white fiber dissection aimed at exposing the superior longitudinal fasciculus, inferior fronto-orbital fasciculus, and uncinate fasciculus. (a) Cross-section image with application of fluorescent solutions but without filters or ultraviolet (UV) light. (b) Shows an image with the application of an alcoholic solution using a gravity-guided technique under only UV light effect. (c) A yellow monochromatic filter is employed to optimize colors and highlight the white fiber tracts.
DISCUSSION
This study is the first of its kind to explore the use of fluorescence in enhancing the visualization and identification of white matter fibers in a neuroanatomy microsurgery laboratory. While numerous studies have been conducted since Kringler’s time to refine the step-by-step dissection of white matter fibers, there has been limited progress in utilizing new tools to guide this practice.[
We propose incorporating fluorescence techniques and monochromatic filters into neurosurgical laboratory training. This approach not only optimizes the practical and theoretical understanding of fluorescence but also enhances the neurosurgeon’s ability to visualize and dissect white matter fibers with greater precision, preparing for a real neurosurgical approach with this useful tool [
The combined use of fiber dissection and fluorescence, particularly when adapted to a gravity-guided technique, provides neurosurgeons with a three-dimensional understanding of both gray and white matter anatomy. This is essential for developing a comprehensive intellectual framework of the brain’s superficial and deep architecture. The application of fluorescence, using UV light and monochromatic filters, offers a more vivid and detailed perspective of brain anatomy.
Expanding on the practical applications, fluorescent agents have increasingly become useful tools in medical diagnostics and interventions. Among them, fluorescein is one of the most commonly used.[
This technique is especially beneficial for trainees, as it deepens their understanding of white fiber tracts and adjacent structures. As we refined our knowledge of white matter anatomy through careful dissections, we also developed the ability to apply this knowledge to post-dissection specimens. This bridging of laboratory training with fluorescence techniques provides a holistic understanding of brain connectivity, aiding in surgical planning and improving outcomes.
Limitations
The availability of suitable brain specimens for dissection can be a significant constraint, impacting the study’s generalizability. Proper specimen preparation requires adequate fixation with formaldehyde, which is not always consistently applied. In addition, the effectiveness of fluorescence techniques in studying white fibers relies heavily on well-fixed specimens. Access to necessary materials, such as fluorescent solutions, UV light, and monochromatic filters, can also be limited despite their low.
CONCLUSION
This study underscores a profound grasp of the fluorescence technique and its pertinent applications in surgical procedures, elucidating the intricacies of tractography imaging creation and its effective utilization in neurosurgical laboratory training, particularly in conjunction with fluorescence. Understanding the clinical applications of tractography is essential, including its use in preoperative planning, evaluating the proximity of critical structures, and predicting potential functional outcomes after surgery. Participating in hands-on exercises that involve generating, interpreting, and comparing tractography images with anatomical dissections helps to reinforce the practical applications of this imaging technique.
We believe that this study will open many doors for the application of fluorescence in training in the neurosurgery laboratory and may have applicability in other fields, such as simulation of infiltrative gliomas that are difficult to differentiate from normal brain parenchyma and by-pass patency testing in animal models or human placentas.
Ethical approval
The research/study was approved by the Institutional Review Board at the Research Ethics Committee of the Federal University of São Paulo, number 56337322.4.0000.5505, dated July 20, 2023.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agrawal A, Kapfhammer JP, Kress A, Wichers H, Deep A, Feindel W. Josef Klingler’s models of white matter tracts: Influences on neuroanatomy, neurosurgery, and neuroimaging. Neurosurgery. 2011. 69: 238-52
2. Choi CY, Han SR, Yee GT, Lee CH. Central core of the cerebrum. J Neurosurg. 2011. 114: 463-9
3. Fernandez-Miranda JC, Pathak S, Engh J, Jarbo K, Verstynen T, Yeh FC. High-definition fiber tractography of the human brain: Neuroanatomical validation and neurosurgical applications. Neurosurgery. 2012. 71: 430-53
4. Fernández-Miranda JC, Rhoton AL, Kakizawa Y, Choi C, Alvarez-Linera J. The claustrum and its projection system in the human brain: A microsurgical and tractographic anatomical study. J Neurosurg. 2008. 108: 764-74
5. Klingler JLudwig E. Atlas cerebri humani. Available from: https://karger.com/book/home/217798 [Last accessed on 2024 Jan 15].
6. Koutsarnakis C, Liakos F, Kalyvas AV, Sakas DE, Stranjalis G. A laboratory manual for stepwise cerebral white matter fiber dissection. World Neurosurg. 2015. 84: 483-93
7. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999. 45: 265-9
8. Soares-Biondi LG, Holanda VM, Lages GV, Soares AG, Catarino MM, Ahumada-Vizcaíno JC. The technique for transorbital ventricular puncture: An anatomic approach. Oper Neurosurg (Hagerstown). 2024. 26: 64-70
9. Wysiadecki G, Clarke E, Polguj M, Haładaj R, Żytkowski A, Topol M. Klingler’s method of brain dissection: Review of the technique including its usefulness in practical neuroanatomy teaching, neurosurgery and neuroimaging. Folia Morphol. 2019. 78: 455-66
10. Zemmoura I, Blanchard E, Raynal PI, Rousselot-Denis C, Destrieux C, Velut S. How Klingler’s dissection permits exploration of brain structural connectivity? An electron microscopy study of human white matter. Brain Struct Funct. 2016. 221: 2477-86