- Department of Neurosurgery, University of New Mexico, University of New Mexico Health Sciences Center, Albuquerque, United States.
Correspondence Address:
Christian A. Bowers, MD, Associate Professor and Vice Chair for Clinical Affairs, Program Director - Neurosurgery Residency, Medical Director -Clinical Neuroscience Center Neurosurgery Clinic, Department of Neurosurgery, University of New Mexico Health Sciences Center, University New Mexico, Albuquerque, NM, 81731, United States.
DOI:10.25259/SNI_542_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kyril L. Cole, Samantha Varela, Kavelin Rumalla, Syed Faraz Kazim, Ryan W. Rebbe, Michael Carvajal, Karen S. SantaCruz, Rohini McKee, Cheryl Willman, Meic H. Schmidt, Christian A. Bowers. Advanced frailty assessment tool predicts successful awake craniotomy in a 92-year-old patient: A case report. 09-Sep-2022;13:404
How to cite this URL: Kyril L. Cole, Samantha Varela, Kavelin Rumalla, Syed Faraz Kazim, Ryan W. Rebbe, Michael Carvajal, Karen S. SantaCruz, Rohini McKee, Cheryl Willman, Meic H. Schmidt, Christian A. Bowers. Advanced frailty assessment tool predicts successful awake craniotomy in a 92-year-old patient: A case report. 09-Sep-2022;13:404. Available from: https://surgicalneurologyint.com/surgicalint-articles/11862/
Abstract
Background: The awake craniotomy (AC) procedure allows for safe and maximal resection of brain tumors from highly eloquent regions. However, geriatric patients are often viewed as poor candidates for AC due to age and medical comorbidities. Frailty assessments gauge physiological reserve for surgery and are valuable tools for preoperative decision-making. Here, we present a novel case illustrating how frailty scoring enabled an elderly but otherwise healthy female to undergo successful AC for tumor resection.
Case Description: A 92-year-old right-handed female with history of hypertension and basal cell skin cancer presented with a 1-month history of progressive aphasia and was found to have a ring-enhancing left frontoparietal mass abutting the rolandic cortex concerning for malignant neoplasm. Frailty scoring with the recalibrated risk analysis index (RAI-C) tool revealed a score of 30 (of 81) indicating low surgical risk. The patient and family were counseled appropriately that, despite advanced chronological age, a low frailty score predicts favorable surgical outcomes. The patient underwent left-sided AC for resection of tumor and experienced immediate improvement of speech intraoperatively. After surgery, the patient was neurologically intact and had an unremarkable postoperative course with significant improvements from preoperatively baseline at follow-up.
Conclusion: To the best of our knowledge, this case represents the oldest patient to undergo successful AC for brain tumor resection. Nonfrail patients over 90 years of age with the proper indications may tolerate cranial surgery. Frailty scoring is a powerful tool for preoperative risk assessment in the geriatric neurosurgery population.
Keywords: Awake craniotomy, Frailty, Malignant tumor, Risk analysis index, Tumor resection
INTRODUCTION
Techniques utilized by neurosurgeons to maximize resection of brain tumors, while minimizing morbidity has improved significantly over time.[
CASE REPORT
History and examination
A 92-year-old right-handed female with a history of hypertension and basal cell skin cancer presented with a 1-month history of progressively worsening speech difficulties. The patient was brought to clinic by her daughter who first noticed the mild changes in sentence syntax. The patient is a retired schoolteacher who at baseline takes no medication, exercises daily, and lives independently. The progressive speech symptoms prompted medical workup including magnetic resonance imaging (MRI) of the brain and subsequent referral to our institution for neurosurgical evaluation. Symptoms at time of consultation included speech difficulties and intermittent right-hand numbness. Neurological examination was positive for expressive aphasia and otherwise unremarkable.
Preoperating neuroimaging
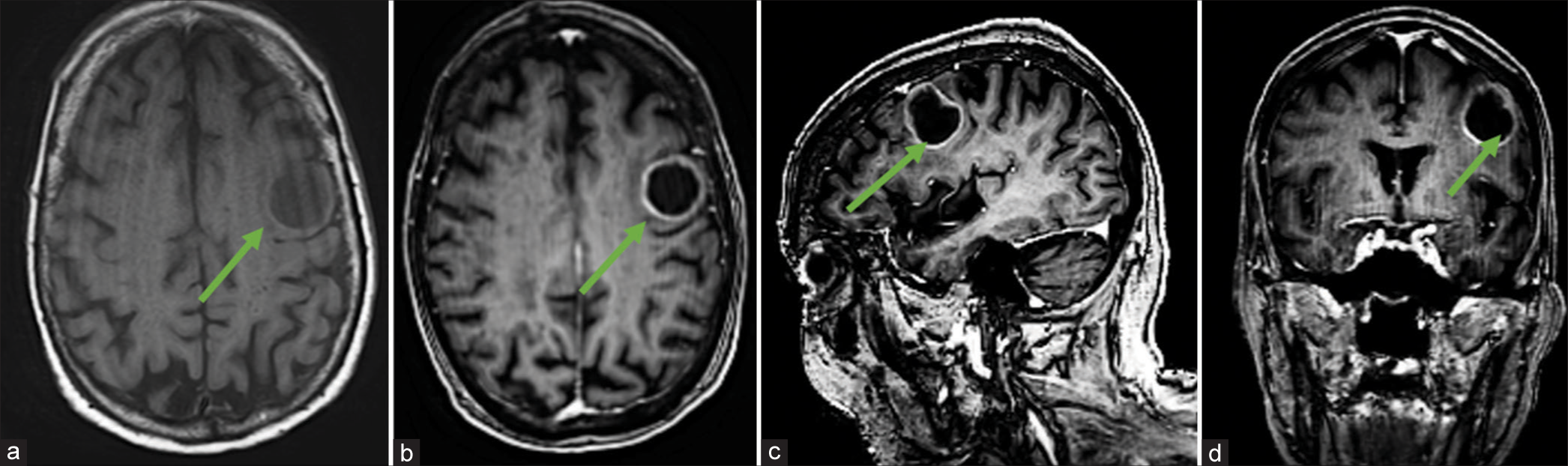
MRI brain with/without contrast revealed a T1 hypointense, rim-enhancing, and intra-axial mass centered in the left precentral gyrus concerning for a malignant neoplasm involving the eloquent rolandic cortex [
Figure 1:
Preoperative neuroimaging. (a) Axial magnetic resonance imaging (MRI)-brain T1 image showing hypointense left frontoparietal mass centered in the left precentral gyrus with Gadolinium-enhanced T1 MRI axial (b), sagittal (c), and coronal (d) images demonstrating rim-enhancement concerning for malignant intracranial neoplasm. Green arrows represent tumor.
Preoperative assessment and patient counseling
The clinical symptoms and imaging findings were strongly suggestive of intermediate to high grade tumor involving the eloquent motor cortex responsible for speech expression. AC would be offered as a primary treatment option given acceptable surgical risk. The risks associated with surgery in the patient’s age group were considered. However, frailty assessment as measured by the recalibrated RAI-C scoring system deemed her a low-risk surgical candidate.[
Operative details
A left-sided frontoparietal temporal AC was performed for resection of tumor with the use of the operative microscope and Medtronic Stealth neuronavigation. Awake speech mapping with intraoperative electrophysiology mapping was also performed with assistance from neuropsychology colleagues. Gross-total resection with achieved without any speech arrest. Speech improved intraoperatively on removal of the cystic portion of tumor.
Histopathological findings
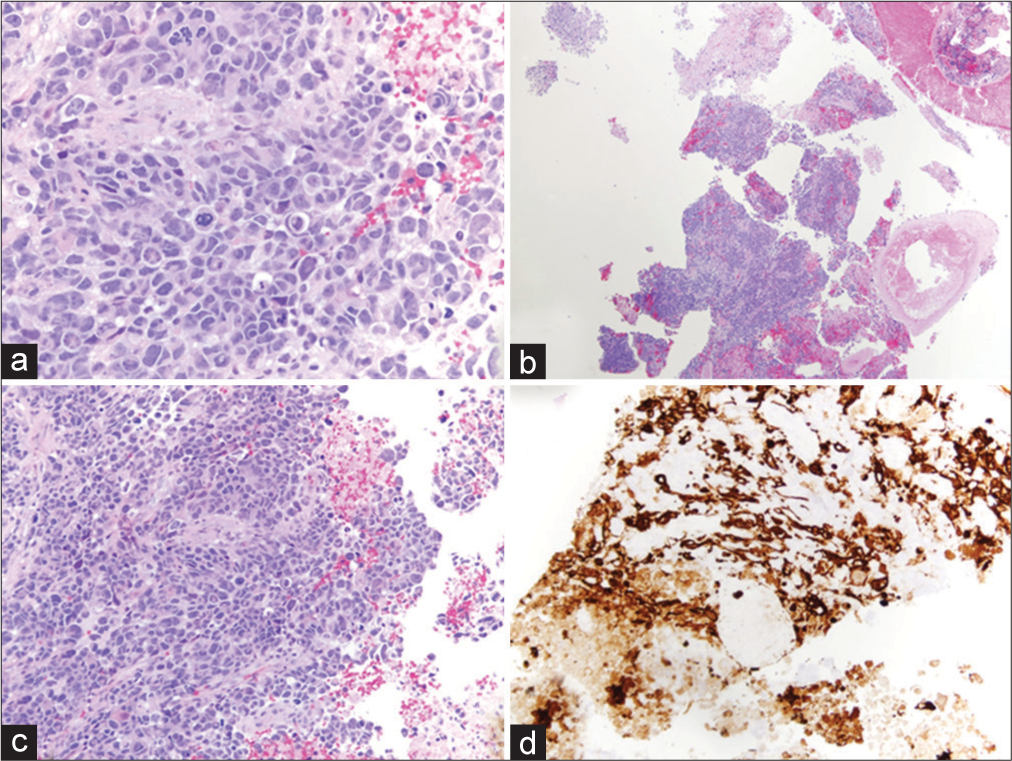
Samples taken during tumor resection were sent to pathology, where microscopic examination of the tissue sections demonstrated a poorly differentiated population of cells with multiforme cytologic features and abundant atypical mitotic figures with vascular proliferation and necrosis, providing a histopathological diagnosis of the WHO Grade IV glioblastoma.
Figure 2:
Histopathology of grade IV glioblastoma multiforme tumor. (a-c) Hematoxylin and eosin staining showing a poorly differentiated population of cells with multiforme cytologic features and some vaguely astrocytic features. There are abundant, often atypical mitotic figures, vascular proliferation, and necrosis. (d) Positive for GFAP immunoreactivity.
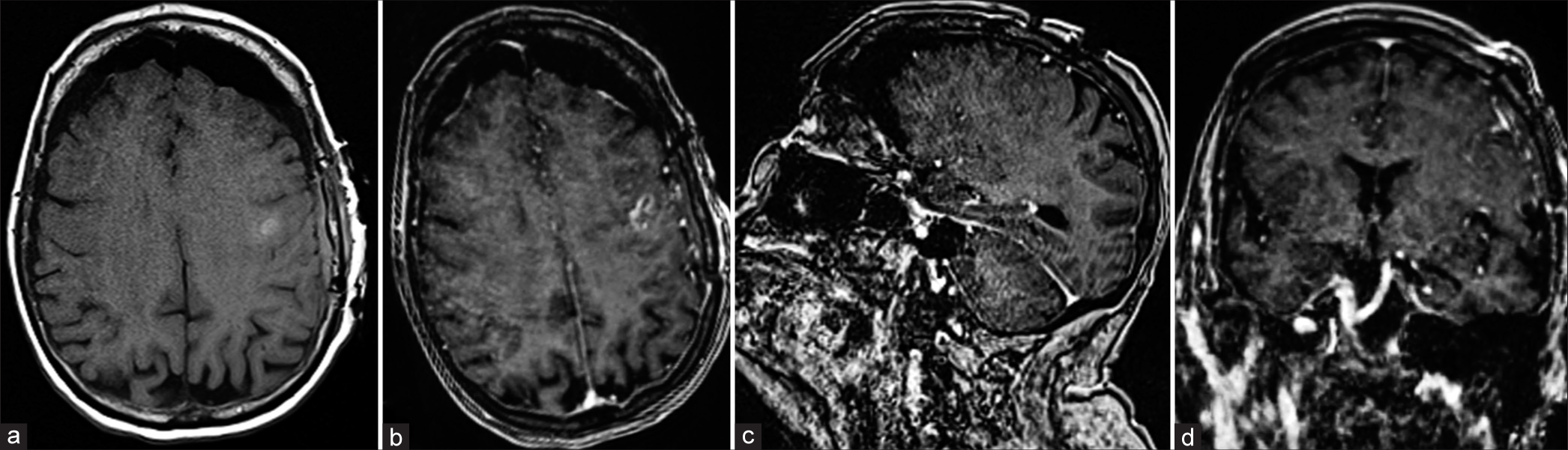
Postoperative imaging and course
Postoperatively, the patient was admitted to the neurosurgical ICU for routine monitoring. The patient was neurologically intact with exception of mild expressive aphasia that improved compared to preoperative assessment. The postoperative MRI with and without contrast was negative for acute complications and showed no residual mass [
DISCUSSION
With current life expectancy trends, octogenarians are expected to triple globally by 2050.[
Based on traditional preoperative evaluations, our patient may have been refused elective AC due to advanced chronological age alone. Similar to chronological age, older comorbidity-based indices[
The RAI is a powerful frailty tool developed and validated to improve the selection of patients for surgery.[
Outcomes data in elderly patients undergoing AC are scarce. A single study by Grossman et al., in 2013, reported outcomes after AC for tumor resection in a series of 334 young (45.4 ± 13.2 years, mean ± SD) and 90 elderly (71.7 ± 5.1 years) patients.[
CONCLUSION
The present case represents the first successful AC for tumor resection in a 92 years old. The case illustrates how robust frailty scoring tools can be integrated into the clinical workflow to select elderly but otherwise healthy patients for surgery. Nonfrail patients over 90 years of age with the proper indications may tolerate cranial surgery. Frailty scoring is a powerful tool for preoperative risk assessment in the geriatric population.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal N, Goldschmidt E, Taylor T, Roy S, Dunn SC, Bilderback A. Impact of frailty on outcomes following spine surgery: A prospective cohort analysis of 668 patients. Neurosurgery. 2021. 88: 552-7
2. Arya S, Varley P, Youk A, Borrebach JD, Perez S, Massarweh NN. Recalibration and external validation of the risk analysis index: A surgical frailty assessment tool. Ann Surg. 2020. 272: 996-1005
3. Bonney PA, Chartrain AG, Briggs RG, Jarvis CA, Ding L, Mack WJ. Frailty is associated with in-hospital morbidity and nonroutine disposition in brain tumor patients undergoing craniotomy. World Neurosurg. 2021. 146: e1045-53
4. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016. 2: 1460-9
5. Cloney M, D’Amico R, Lebovic J, Nazarian M, Zacharia BE, Sisti MB. Frailty in geriatric glioblastoma patients: A predictor of operative morbidity and outcome. World Neurosurg. 2016. 89: 362-7
6. D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: The Charlson comorbidity index. Methods Inf Med. 1993. 32: 382-7
7. De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010. 66: 1074-84
8. Dicpinigaitis AJ, Hanft S, Cooper JB, Gandhi CD, Kazim SF, Schmidt MH. Comparative associations of baseline frailty status and age with postoperative mortality and duration of hospital stay following metastatic brain tumor resection. Clin Exp Metastasis. 2022. 39: 303-10
9. Dicpinigaitis AJ, Kalakoti P, Schmidt M, Gurgel R, Cole C, Carlson A. Associations of baseline frailty status and age with outcomes in patients undergoing vestibular schwannoma resection. JAMA Otolaryngol Head Neck Surg. 2021. 147: 608-14
10. Dicpinigaitis AJ, Kazim SF, Schmidt MH, Couldwell WT, Theriault BC, Gandhi CD. Association of baseline frailty status and age with postoperative morbidity and mortality following intracranial meningioma resection. J Neurooncol. 2021. 155: 45-52
11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998. 36: 8-27
12. Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003. 238: 170-7
13. Grossman R, Nossek E, Sitt R, Hayat D, Shahar T, Barzilai O. Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann Surg Oncol. 2013. 20: 1722-8
14. Hall DE, Arya S, Schmid KK, Blaser C, Carlson MA, Bailey TL. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017. 152: 175-82
15. Harland TA, Wang M, Gunaydin D, Fringuello A, Freeman J, Hosokawa PW. Frailty as a predictor of neurosurgical outcomes in brain tumor patients. World Neurosurg. 2020. 133: e813-8
16. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low-and high-grade glioma. J Neurooncol. 2016. 130: 269-82
17. Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J Neurosurg. 2015. 123: 325-39
18. Huq S, Khalafallah AM, Jimenez AE, Gami A, Lam S, RuizCardozo MA. Predicting postoperative outcomes in brain tumor patients with a 5-factor modified frailty index. Neurosurgery. 2021. 88: 147-54
19. Huq S, Khalafallah AM, Jimenez AE, Gami A, Lam S, RuizCardozo MA. Predicting postoperative outcomes in brain tumor patients with a 5-factor modified frailty index. Neurosurgery. 2020. 88: 147-54
20. Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008. 451: 716-9
21. McIsaac DI, Aucoin SD, van Walraven C. A Bayesian comparison of frailty instruments in noncardiac surgery: A cohort study. Anesth Analg. 2021. 133: 366-73
22. Patil CG, Veeravagu A, Lad SP, Boakye M. Craniotomy for resection of meningioma in the elderly: a multicentre, prospective analysis from the National Surgical Quality Improvement Program. J Neurol Neurosurg Psychiatry. 2010. 81: 502-5
23. Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery. 2011. 68: 1192-8
24. Sastry RA, Pertsch NJ, Tang O, Shao B, Toms SA, Weil RJ. Frailty and outcomes after craniotomy for brain tumor. J Clin Neurosci. 2020. 81: 95-100
25. Schär RT, Tashi S, Branca M, Söll N, Cipriani D, Schwarz C. How safe are elective craniotomies in elderly patients in neurosurgery today? A prospective cohort study of 1452, consecutive cases. J Neurosurg. 2020. 134: 1113-21
26. Shah R, Borrebach JD, Hodges JC, Varley PR, Wisniewski MK, Shinall MC. Validation of the risk analysis index for evaluating frailty in ambulatory patients. J Am Geriatr Soc. 2020. 68: 1818-24
27. Tachibana S, Omote M, Yamakage M. Successful awake craniotomy in an aged patient with a severe hearing impairment using a bone conduction voice amplifier: A case report. JA Clin Rep. 2019. 5: 37
28. Theriault BC, Pazniokas J, Adkoli AS, Cho EK, Rao N, Schmidt M. Frailty predicts worse outcomes after intracranial meningioma surgery irrespective of existing prognostic factors. Neurosurgical Focus. 2020. 49: E16
29. Thommen R, Kazim SF, Cole KL, Olson GT, Shama L, Lovato CM. Worse pituitary adenoma surgical outcomes predicted by increasing frailty, not age. World Neurosurg. 2022. 161: e347-54
30. Varley PR, Borrebach JD, Arya S, Massarweh NN, Bilderback AL, Wisniewski MK. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2021. 274: e1230-7
31. Walston J, Bandeen-Roche K, Buta B, Bergman H, Gill TM, Morley JE. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc. 2019. 67: 1559-64