- Emergency and Critical Care Center, Kawaguchi Municipal Medical Center, Kawaguchi-shi, Saitama, Japan
- Department of Emergency and Critical Care Medicine, Nippon Medical School, Bunkyo-ku, Tokyo, Japan
Correspondence Address:
Ryuta Nakae
Department of Emergency and Critical Care Medicine, Nippon Medical School, Bunkyo-ku, Tokyo, Japan
DOI:10.4103/sni.sni_56_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryuta Nakae, Shoji Yokobori, Yasuhiro Takayama, Kentaro Kuwamoto, Yasutaka Naoe, Hiroyuki Yokota. Age-related differences in fibrinolytic parameters in patients with acute traumatic brain injury. 06-Sep-2017;8:214
How to cite this URL: Ryuta Nakae, Shoji Yokobori, Yasuhiro Takayama, Kentaro Kuwamoto, Yasutaka Naoe, Hiroyuki Yokota. Age-related differences in fibrinolytic parameters in patients with acute traumatic brain injury. 06-Sep-2017;8:214. Available from: http://surgicalneurologyint.com/surgicalint-articles/age%e2%80%91related-differences-in-fibrinolytic-parameters-in-patients-with-acute-traumatic-brain-injury/
Abstract
Background:Coagulopathy and old age have been associated with poor outcomes in traumatic brain injury (TBI) patients; however, the relationships of coagulopathy and age with the acute phase of TBI remain unclear. We hypothesized that coagulation/fibrinolytic abnormalities are more severe in older patients in the acute phase of TBI and may explain, in part, their poor outcome.
Methods:We analyzed the relationship between coagulation/fibrinolytic parameters and age in the acute phase of TBI by retrospectively evaluating 274 patients with initial blood samples obtained no more than 1 hour after injury. Measurement of platelet count, prothrombin time, activated partial thromboplastin time, plasma levels of fibrinogen, and D-dimer was done in the emergency department on arrival as well as 3, 6, and 12 hours following injury. Values were compared between patients aged 16–55 years (group 1) and those aged older than 55 years (group 2) with an Abbreviated Injury Score (AIS)-head of 3–5 to identify any relationship between these parameters and age.
Results:When groups 1 and 2 were matched for AIS-head, plasma levels of D-dimer in group 2 were significantly higher than those in group 1 from hospital admission to 12 hours after injury. The Glasgow Outcome Scale scores at 3 months post-injury of group 2 with AIS 4 and 5 were significantly lower than those of group 1 (both P
Conclusions:Fibrinolytic abnormalities are more severe in older acute-phase TBI patients, which may be a factor associated with their poor prognosis.
Keywords: Age, coagulopathy, D-dimer, fibrinolysis, traumatic brain injury
INTRODUCTION
Increased age is associated with a poor outcome in patients with traumatic brain injury (TBI).[
MATERIALS AND METHODS
Setting
The Emergency and Critical Care Center of Kawaguchi Municipal Medical Center (Saitama, Japan) is a major critical care center in Saitama Prefecture which treats approximately 1000–1200 patients per year, including 300–350 patients with severe trauma.
Patients
We retrospectively evaluated the demographic, clinical, and radiologic data of consecutive patients with TBI admitted to the center from April 2007 to June 2015. Patients with a diagnosis of severe isolated TBI were eligible, as defined previously as an intracranial Abbreviated Injury Score (AIS-head) ≥3 and extracranial AIS <3.[
Information on factors influencing coagulation and fibrinolytic parameters was accessed, such as age,[
Management of traumatic brain injury
Treatment was given immediately on arrival at the emergency department according to the guidelines for management of TBI developed by the Japan Society of Neurotraumatology.[
Assay of coagulation/fibrinolytic parameters
Blood samples were drawn into ethylenediaminetetraacetic acid (EDTA) and citrate vacuum tubes. Platelet count was determined by the DC sheath flow detection method (Cellpack II® and SE sheath II®, Sysmex Corp., Kobe, Japan). PT was measured by the coagulation method (Dade Innovin®, Sysmex Corp., Kobe, Japan). APTT was quantified by the coagulation method (Thrombocheck APTT-SLA®, Sysmex Corp., Kobe, Japan). Fibrinogen was determined by the coagulation method (Thrombocheck Fib (L)®, Sysmex Corp., Kobe, Japan). D-dimer was measured by the latex immunoassay method (LIAS Auto D-dimer Neo®, Sysmex Corp., Kobe, Japan).
Statistical analysis
Data were analyzed using commercial software (SPSS Version 20.0®; IBM Corp., Armonk NY, USA). Patients were categorized by age into two groups, with Group 1 including patients aged 16–55 years and Group 2 including those aged >55 years. This age threshold was chosen based on a report of the United States Traumatic Coma Data Bank.[
To evaluate the relationship of coagulation/fibrinolytic parameters to age alone, patients were divided into three groups according to AIS-head. We compared coagulation/fibrinolytic parameters between group 1 and group 2 in AIS 3 patients, AIS 4 patients, and AIS 5 patients.
RESULTS
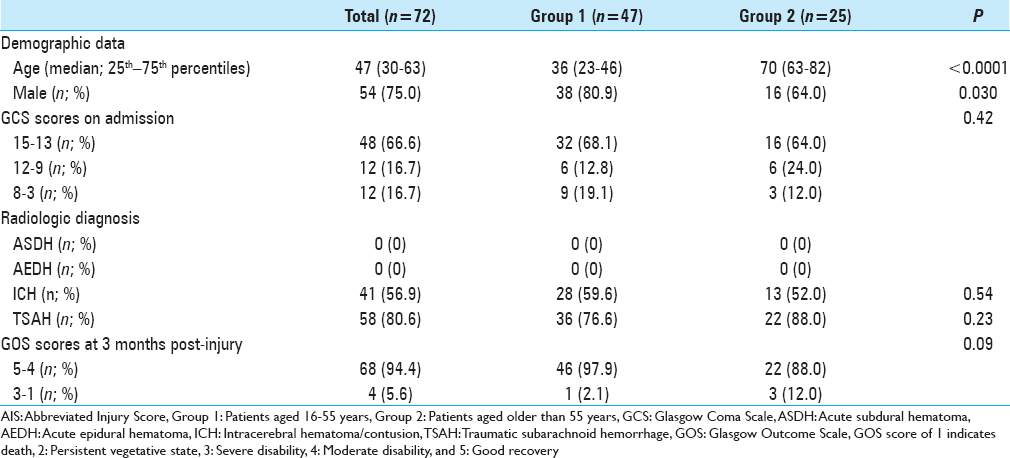
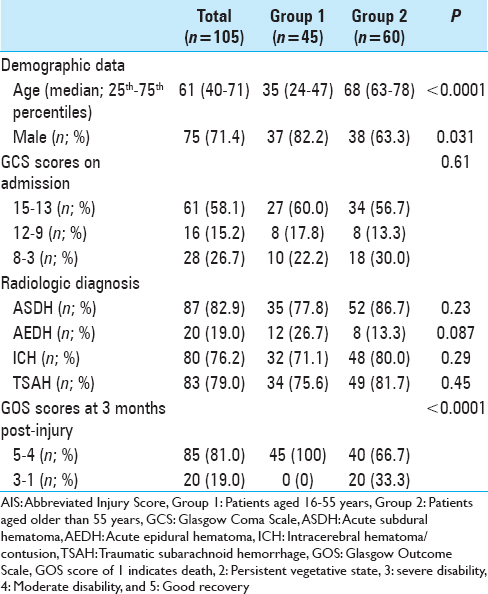
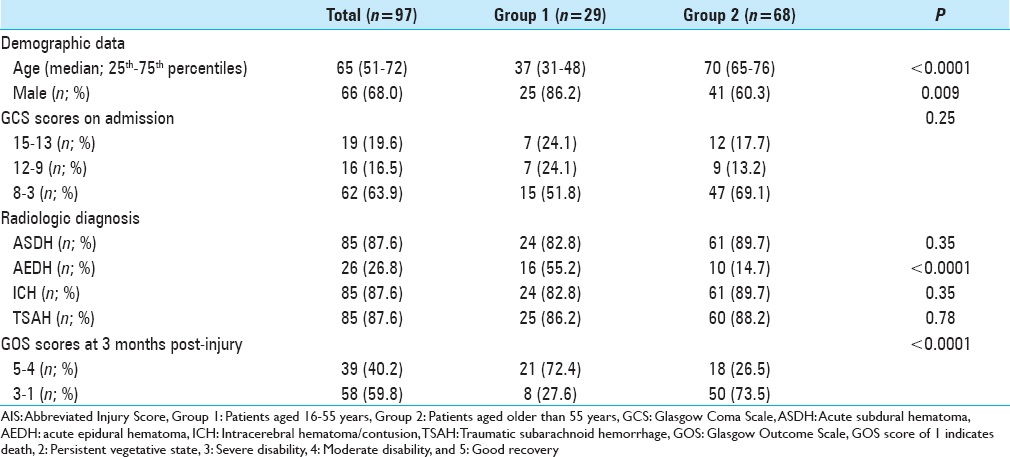
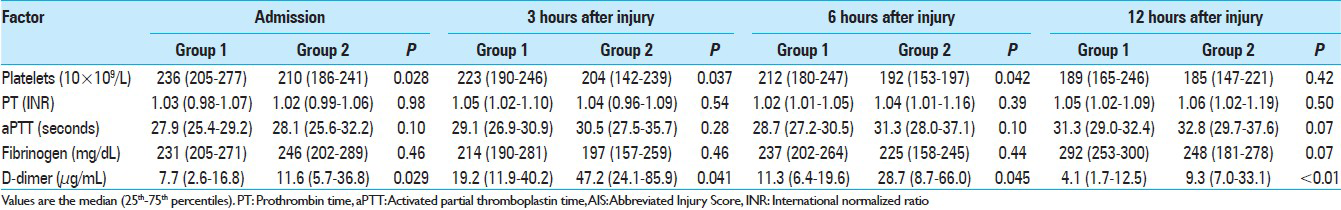
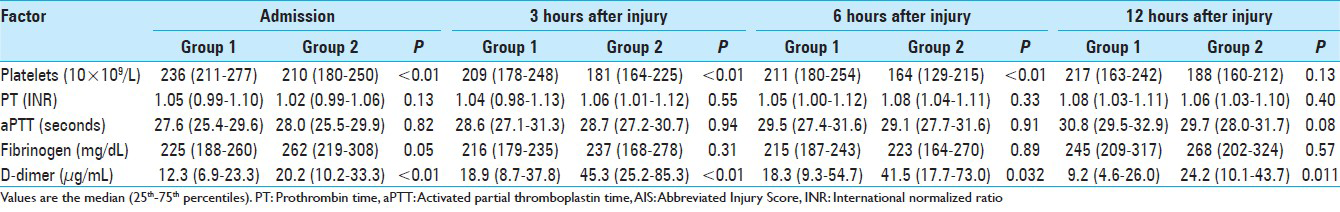
ASDH was observed in 172 (62.8%) patients, AEDH in 46 (16.8%), ICH in 206 (75.2%), and TSAH in 226 (82.5%) (some patients had more than one diagnosis). Demographic, clinical, and radiologic diagnoses of the patients with AIS 3–5 are summarized in Tables
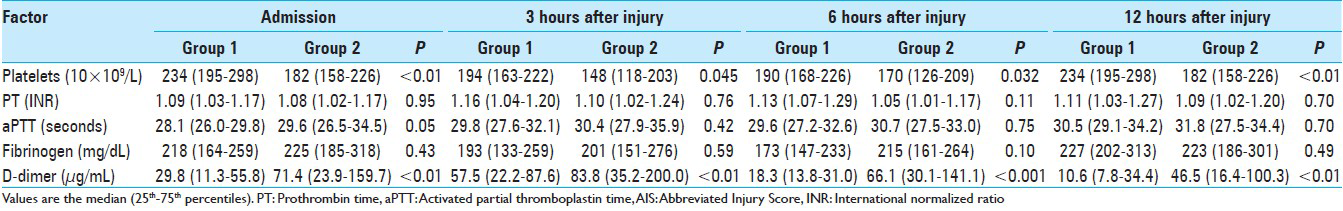
Blood samples for coagulation and fibrinolytic parameters were collected at 0.65 ± 0.19, 3.04 ± 0.12, 6.05 ± 0.17, and 12.05 ± 0.21 hours after injury for the first, second, third, and fourth sets of laboratory values, respectively. Figures
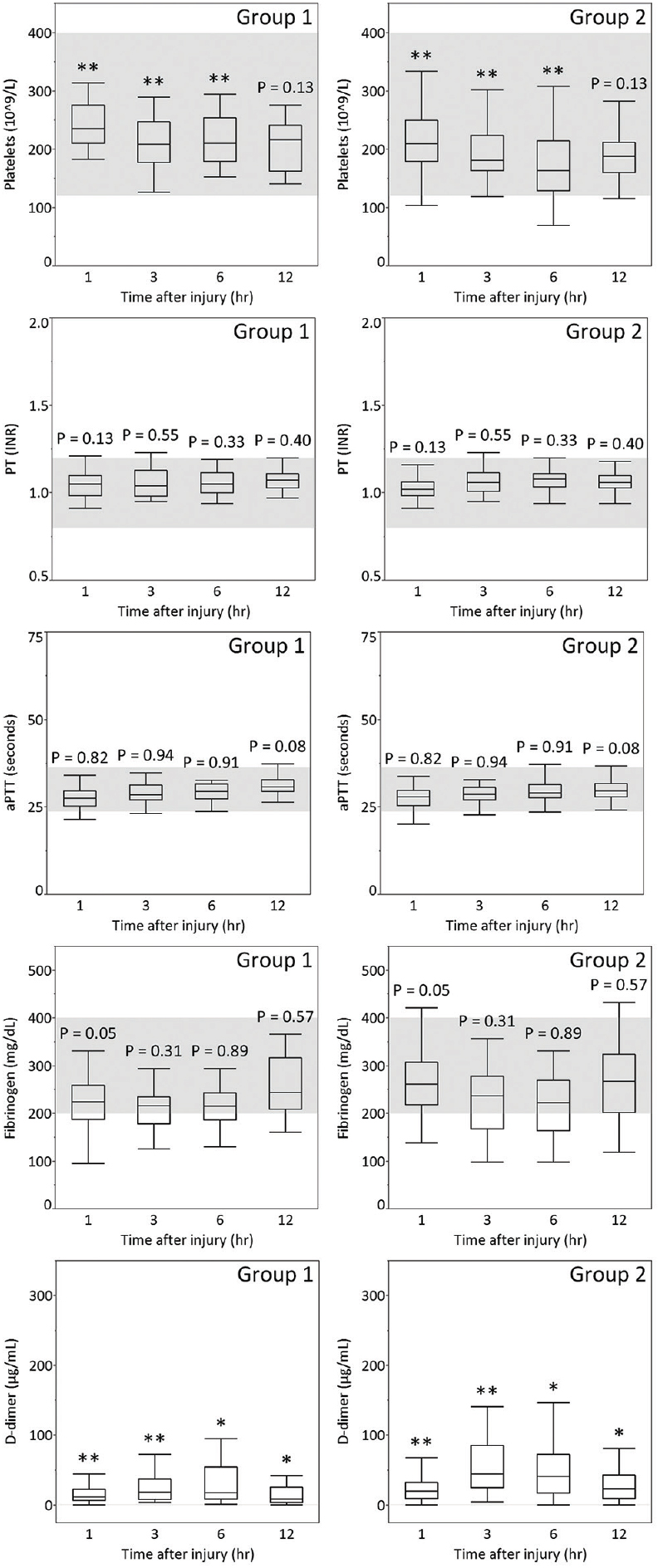
Figure 1
Platelet count, PT, aPTT, and plasma levels of fibrinogen and D-dimer of patients aged 16–55 years (Group 1) or aged older than 55 years (Group 2) with AIS-head 3 on admission and at 3, 6, and 12 hours after TBI. The gray zones represent the normal ranges for each parameter. *P < 0.05, **P < 0.01
Figure 2
Platelet count, PT, aPTT, and plasma levels of fibrinogen and D-dimer of patients aged 16–55 years (Group 1) or aged older than 55 years (Group 2) with AIS-head 4 on admission and at 3, 6, and 12 hours after TBI. The gray zones represent the normal ranges for each parameter. *P < 0.05, **P < 0.01
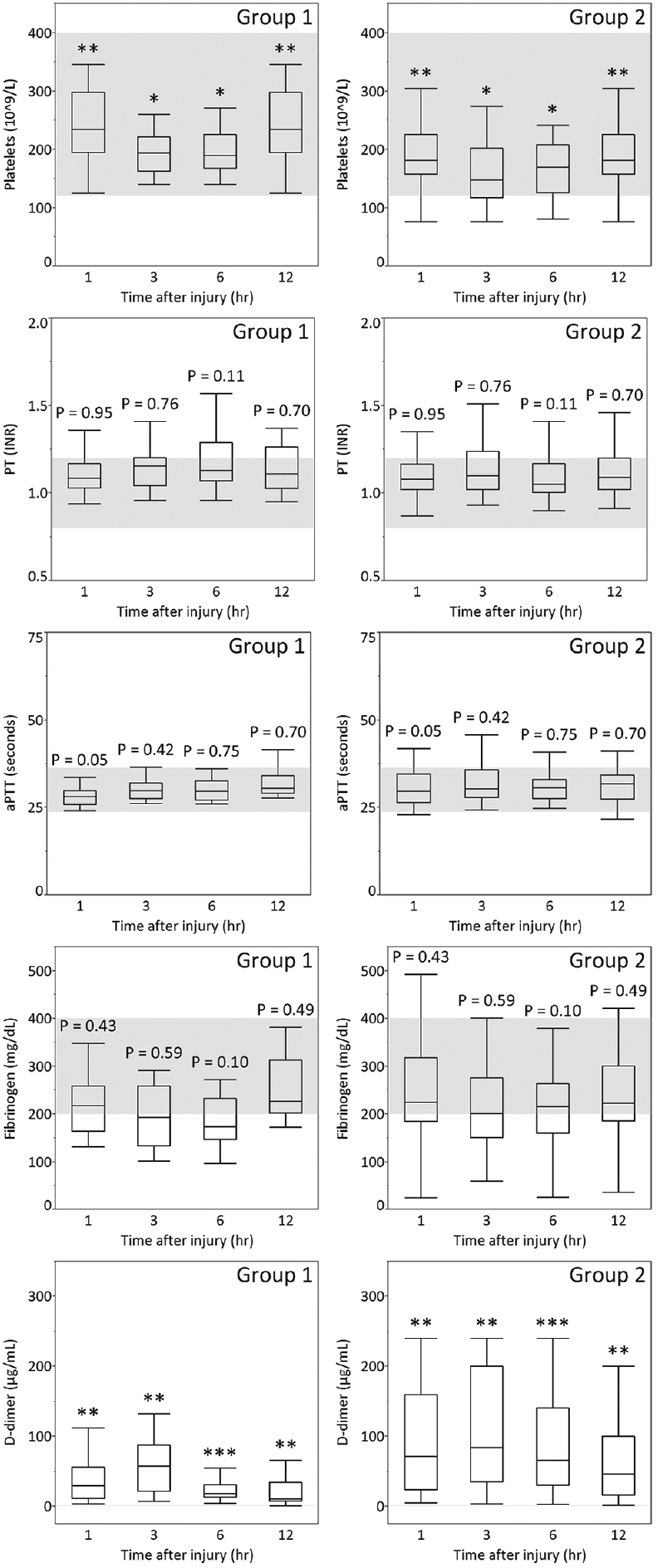
Figure 3
Platelet count, PT, aPTT, and plasma levels of fibrinogen and D-dimer of patients aged 16–55 years (Group 1) or aged older than 55 years (Group 2) with AIS-head 5 on admission and at 3, 6, and 12 hours after TBI. The gray zones represent the normal ranges for each parameter. *P < 0.05, **P < 0.01, ***P < 0.001
DISCUSSION
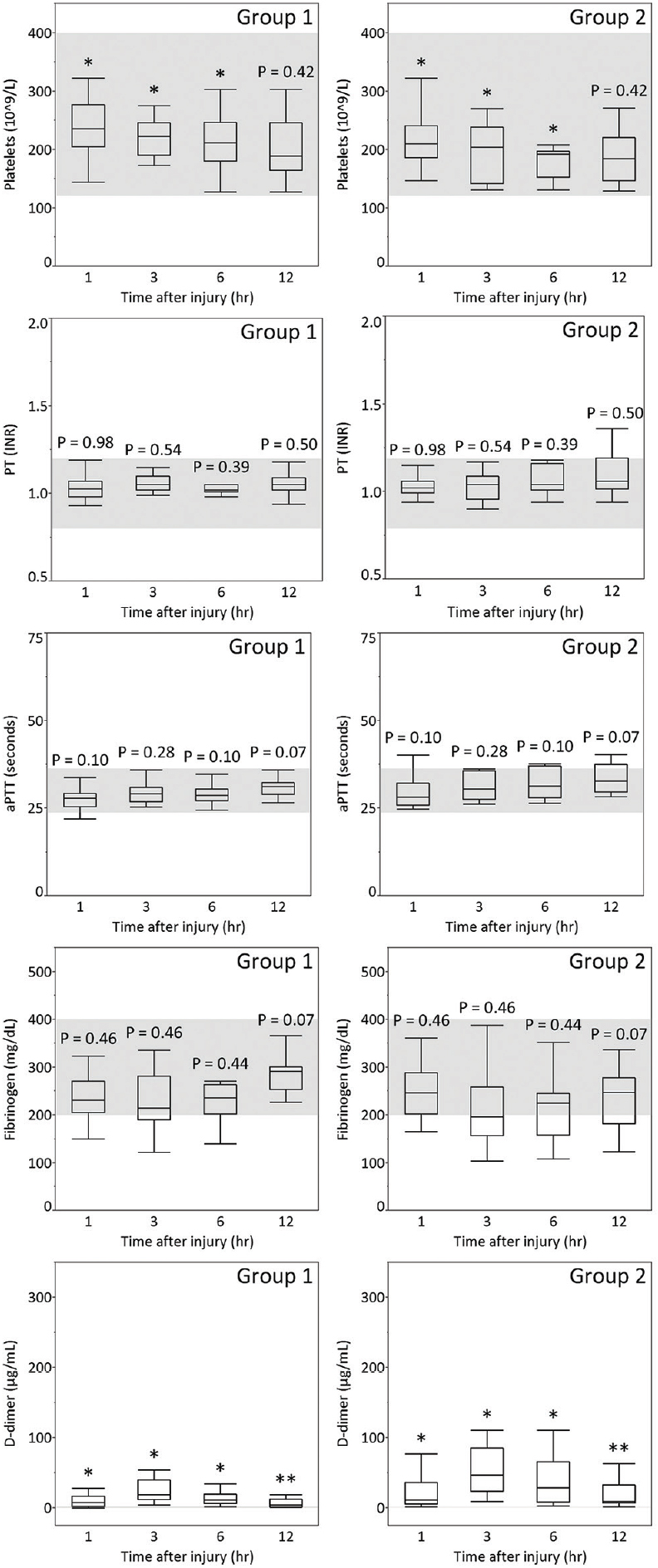
In this analysis of the time course of coagulation and fibrinolytic parameters in the acute phase of TBI, we found that coagulation parameters such as platelet count and fibrinolytic parameters such as D-dimer differed more strongly between groups 1 and 2 than coagulation parameters such as PT, aPTT, and fibrinogen. In particular, D-dimer showed age-related differences at all time points in each AIS-head of 3 to 5 from admission to 12 hours after injury.
Several studies have demonstrated that platelet count decreases with age.[
The early hypercoagulable state after TBI is followed by an increase in fibrinolytic activity, with fibrinolytic parameters such as fibrin/fibrinogen degradation products (FDP) and D-dimer being reliable prognostic markers.[
We observed that the median plasma level of D-dimer was higher in group 2 than in group 1 in AIS-head matched patients from admission to 12 hours after injury [Figures
D-dimer as a fibrinolytic parameter progressively increased from admission to 3 hours after injury and remained raised for at least a further 12 hours, indicating that presentation within 12 hours after injury carries a high risk of exacerbation of hemorrhage due to hyperfibrinolysis, especially within 3 hours after injury. Allard et al. reported that mortality increased four-fold (32% vs. 8%) on follow-up CT scan in coagulopathic patients with hemorrhagic progression.[
Our results suggest that the correction of coagulopathy caused by hyperfibrinolysis may be especially important in the treatment of geriatric TBI. The Clinical Randomisation of Antifibrinolytic in Significant Hemorrhage (CRASH-2) trial is a major international multicenter randomized placebo-controlled study of the impact of the antifibrinolytic agent tranexamic acid (TXA) on death and requirements for transfusion in adult trauma patients suffering significant hemorrhage.[
Limitations
Several limitations of our study warrant mention. First, the study was not designed to investigate the effect of surgery or hemodilution due to osmotic diuretic agents, which might affect coagulation or fibrinolytic variables at 3, 6 and 12 hours post-injury. Second, differences in the mechanism of TBI between Japan and other countries are probably due to the high mean age of Japanese, which would result in an apparent overrepresentation of older patients with severe TBI. Calculation of the GOS score at 3 months may be somewhat early with severe TBI. Evaluating of recovery after severe TBI needs ongoing investigation and implementation of long-term follow-up.
CONCLUSIONS
Fibrinolytic abnormalities are more severe in older TBI patients in the acute phase, and appear to be one of the reasons why older TBI patients have a poor outcome. Future studies should reveal whether early recognition of acute coagulopathy and the prevention of delayed hemostatic disturbances might be associated with improvements in morbidity and mortality in older TBI patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Allard CB, Scarpelini S, Rhind SG, Baker AJ, Shek PN, Tien H. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J Trauma. 2009. 67: 959-67
2. .editorsAssociation for the Advancement of Automotive Medicine. The Abbreviated Injury Scale (AIS) 1998 Revision. Des Plaines, Illinois: Association for the Advancement of Automotive Medicine; 1998. p.
3. Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M. Age- and sex-related variations in platelet count in Italy: A proposal of reference ranges based on 40987 subjects’ data. PloS One. 2013. 8: e54289-
4. Cadroy Y, Pierrejean D, Fontan B, Sie P, Boneu B. Influence of aging on the activity of the hemostatic system: Prothrombin fragment 1+2, thrombin-antithrombin III complexes and D-dimers in 80 healthy subjects with age ranging from 20 to 94 years. Nouv Rev Fr Hematol. 1992. 34: 43-6
5. Carrick MM, Tyroch AH, Youens CA, Handley T. Subsequent development of thrombocytopenia and coagulopathy in moderate and severe head injury: Support for serial laboratory examination. J Trauma. 2005. 58: 725-
6. Accessed on 2017 Feb 5. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01990768#contacts.
7. . CRASH-2 Trial Collaborators. Effect of tranexamic acid in traumatic brain injury: A nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ. 2011. 343: d3795-
8. . CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet. 2010. 376: 23-32
9. Crone KR, Lee KS, Kelly DL. Correlation of admission fibrin degradation products with outcome and respiratory failure in patients with severe head injury. Neurosurgery. 1987. 21: 532-6
10. Currie MS, Rao MK, Blazer DG, Cohen HJ. Age and functional correlations of markers of coagulation and inflammation in the elderly: Functional implications of elevated crosslinked fibrin degradation products (D-dimers). J Am Geriatr Soc. 1994. 42: 738-42
11. Dewan Y, Komolafe EO, Mejia-Mantilla JH, Perel P, Roberts I, Shakur H. CRASH-3-tranexamic acid for the treatment of significant traumatic brain injury: Study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012. 13: 87-
12. Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: A systematic review and meta-analysis. Injury. 2014. 45: 819-24
13. Favaloro EJ, Franchini M, Lippi G. Aging hemostasis: Changes to laboratory markers of hemostasis as we age-a narrative review. Semin Thromb Hemost. 2014. 40: 621-33
14. Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006. 60: 144-51
15. Gando S, Nanzaki S, Kemmotsu O. Coagulofibrinolytic changes after isolated head injury are not different from those in trauma patients without head injury. J Trauma. 1999. 46: 1070-
16. Greuters S, van den Berg A, Franschman G, Viersen VA, Beishuizen A, Peerdeman SM. Acute and delayed mild coagulopathy are related to outcome in patients with isolated traumatic brain injury. Crit Care. 2011. 15: R2-
17. Hager K, Platt D. Fibrin degeneration product concentrations (D-dimers) in the course of ageing. Gerontology. 1995. 41: 159-65
18. Halpern CH, Reilly PM, Turtz AR, Stein SC. Traumatic coagulopathy: The effect of brain injury. J Neurotrauma. 2008. 25: 997-1001
19. Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien). 2008. 150: 165-75
20. Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF. Patient age and outcome following severe traumatic brain injury: An analysis of 5600 patients. J Neurosurg. 2003. 99: 666-73
21. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981. 44: 285-93
22. Juratli TA, Zang B, Litz RJ, Sitoci KH, Aschenbrenner U, Gottschlich B. Early hemorrhagic progression of traumatic brain contusions: Frequency, correlation with coagulation disorders, and patient outcome: A prospective study. J Neurotrauma. 2014. 31: 1521-7
23. Kario K, Matsuo T, Kobayashi H. Which factors affect high D-dimer levels in the elderly?. Thromb Res. 1991. 62: 501-8
24. Kuo JR, Chou TJ, Chio CC. Coagulopathy as a parameter to predict the outcome in head injury patients-analysis of 61 cases. J Clin Neurosci. 2004. 11: 710-4
25. Lee AJ, Fowkes GR, Lowe GD, Rumley A. Determinants of fibrin D-dimer in the Edinburgh Artery Study. Arterioscler Thromb Vasc Biol. 1995. 15: 1094-7
26. Lustenberger T, Talving P, Kobayashi L, Inaba K, Lam L, Plurad D. Time course of coagulopathy in isolated severe traumatic brain injury. Injury. 2010. 41: 924-8
27. Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008. 43: 66-73
28. Mari D, Mannucci PM, Coppola R, Bottasso B, Bauer KA, Rosenberg RD. Hypercoagulability in centenarians: The paradox of successful aging. Blood. 1995. 85: 3144-9
29. Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI. Multivariable prognostic analysis in traumatic brain injury: Results from the IMPACT study. J Neurotrauma. 2007. 24: 329-37
30. Nakae R, Takayama Y, Kuwamoto K, Naoe Y, Sato H, Yokota H. Time Course of Coagulation and Fibrinolytic Parameters in Patients with Traumatic Brain Injury. J Neurotrauma. 2016. 33: 688-95
31. Olson JD, Kaufman HH, Moake J, O’Gorman TW, Hoots K, Wagner K. The incidence and significance of hemostatic abnormalities in patients with head injuries. Neurosurgery. 1989. 24: 825-32
32. Pieper CF, Rao KM, Currie MS, Harris TB, Cohen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000. 55: M649-57
33. Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006. 16: 123-30
34. Shigemori M, Abe T, Aruga T, Ogawa T, Okudera H, Ono J. Guidelines for the Management of Severe Head Injury, 2nd Edition guidelines from the Guidelines Committee on the Management of Severe Head Injury, the Japan Society of Neurotraumatology. Neurol Med Chir (Tokyo). 2012. 52: 1-30
35. Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit care. 2004. 1: 479-88
36. Stein SC, Spettell C, Young G, Ross SE. Delayed and progressive brain injury in closed-head trauma: Radiological demonstration. Neurosurgery. 1993. 32: 25-
37. Takahashi H, Urano T, Takada Y, Nagai N, Takada A. Fibrinolytic parameters as an admission prognostic marker of head injury in patients who talk and deteriorate. J Neurosurg. 1997. 86: 768-72
38. Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan LS, Demetriades D. Coagulopathy in severe traumatic brain injury: A prospective study. J Trauma. 2009. 66: 55-
39. Tita-Nwa F, Bos A, Adjei A, Ershler WB, Longo DL, Ferrucci L. Correlates of D-dimer in older persons. Aging Clin Exp Res. 2010. 22: 20-3
40. Troussard X, Vol S, Cornet E, Bardet V, Couaillac JP, Fossat C. Full blood count normal reference values for adults in France. J Clin Pathol. 2014. 67: 341-4
41. Vollmer DG, Torner JC, Jane JA, Sadovnic B, Charlebois D, Eisenberg HM. Age and outcome following traumatic coma: Why do older patients fare worse?. J Neurosurg. 1991. 75: S37-S49
42. Wafaisade A, Lefering R, Tjardes T, Wutzler S, Simanski C, Paffrath T. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocritical care. 2010. 12: 211-9
43. Yokota H, Atsumi T, Araki T, Fuse A, Sato H, Kushimoto S. Cerebral endothelial injury in elderly patients with severe head injury measured by serum thrombomodulin and von Willebrand factor. Neurol Med Chir (Tokyo). 2007. 47: 383-8
44. Yokota H, Naoe Y, Nakabayashi M, Unemoto K, Kushimoto S, Kurokawa A. Cerebral endothelial injury in severe head injury: The significance of measurements of serum thrombomodulin and the von Willebrand factor. J Neurotrauma. 2002. 19: 1007-15