- Department of Neurosurgery, Medical Center East, Tokyo Women’s Medical University, Tokyo, Japan.
DOI:10.25259/SNI_271_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Ebise, Yuichi Kubota, Hidenori Ohbuchi, Naoyuki Arai, Mayuko Inazuka, Mikhail Chernov, Hidetoshi Kasuya. Aggressive internal and external decompression as a life-saving surgery in a deeply comatose patient with fixed dilated pupils after severe traumatic brain injury: A case report. 11-Jul-2020;11:181
How to cite this URL: Hiroki Ebise, Yuichi Kubota, Hidenori Ohbuchi, Naoyuki Arai, Mayuko Inazuka, Mikhail Chernov, Hidetoshi Kasuya. Aggressive internal and external decompression as a life-saving surgery in a deeply comatose patient with fixed dilated pupils after severe traumatic brain injury: A case report. 11-Jul-2020;11:181. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10123
Abstract
Background: To maximize control of the intracranial pressure in deeply comatose patients with malignant cerebral swelling, combination of the surgical techniques for internal and external brain decompression may be reasonable, as demonstrated in the presented case.
Case Description: A 55-year-old man was admitted with Glasgow Coma Scale (GCS) score 4, maximally dilated pupils, and absence of the pupillary light and vestibulo-ocular reflexes. Head CT revealed massive acute subdural hematoma, prominent brain shift with subfalcine and transtentorial herniation, and diffuse subarachnoid hemorrhage. Large size decompressive craniectomy and evacuation of subdural hematoma were done, however, prominent swelling of the brain and its protrusion through the bone defect remained. Therefore, extensive temporal lobectomy and removal of the bulk of temporal muscle were additionally attained followed by lax duraplasty. Gradual recovery of the patient was noted from the 1st postoperative day, and on the 70th day, his GCS score was 4T4. Three months later, his condition corresponded to the Glasgow Outcome Scale score 3 (severe disability).
Conclusion: Aggressive internal and external decompression with combination of large size craniectomy, extensive temporal lobectomy, removal of the bulk of temporal muscle, and lax duraplasty should be considered as possible life-saving option in cases of neurosurgical emergencies with malignant cerebral swelling.
Keywords: Decompressive craniectomy, Malignant cerebral swelling, Severe traumatic brain injury, Temporal lobectomy, Temporal muscle resection, Transtentorial herniation
INTRODUCTION
Extremely large size craniectomy, sometimes referring as hemicraniectomy, has been applied widely for external decompression to control intracranial pressure (ICP) in cases of extensive hemispheric infarction, severe traumatic brain injury, and other neurological and neurosurgical emergencies accompanied by malignant cerebral swelling.[
CASE DESCRIPTION
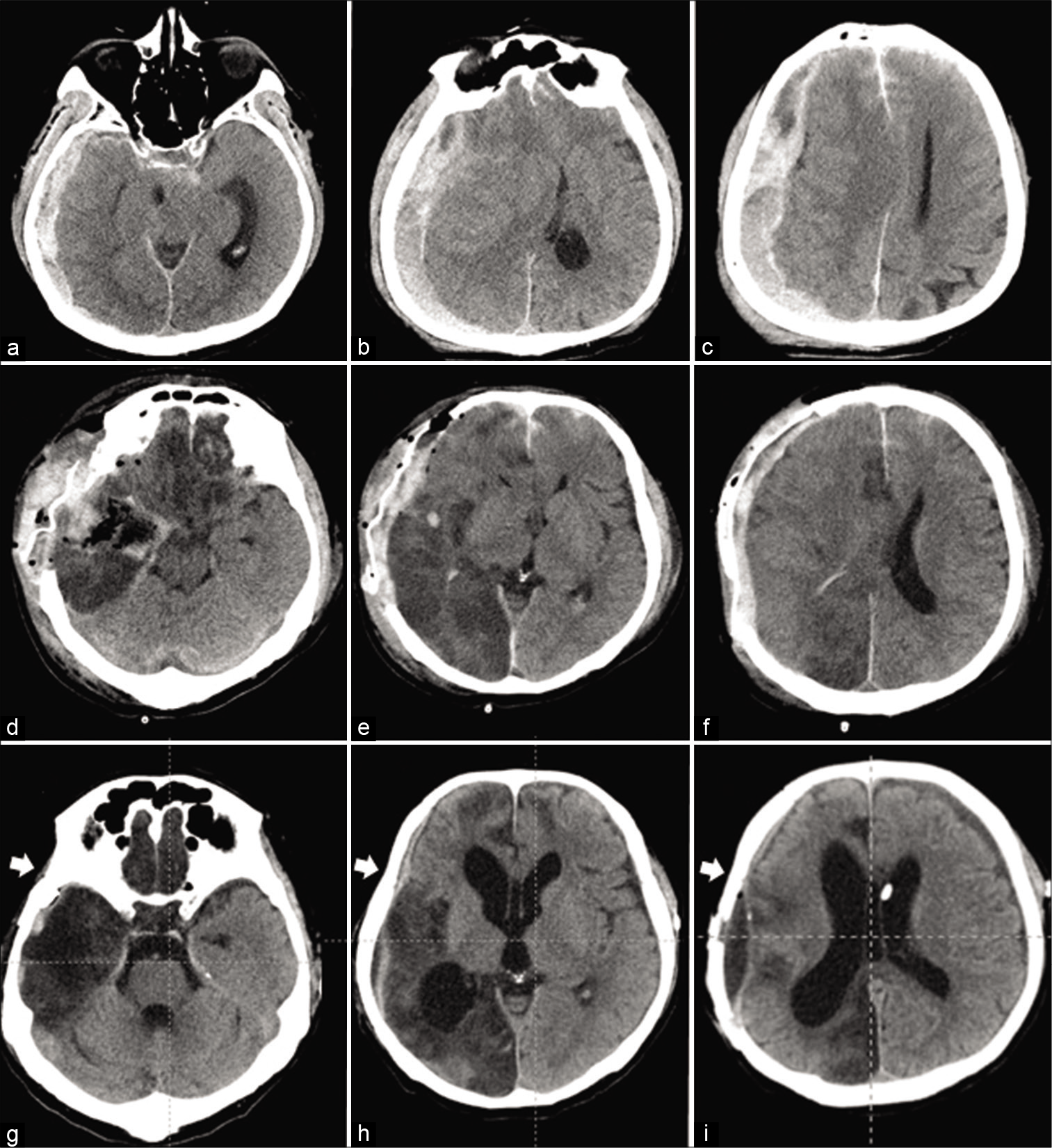
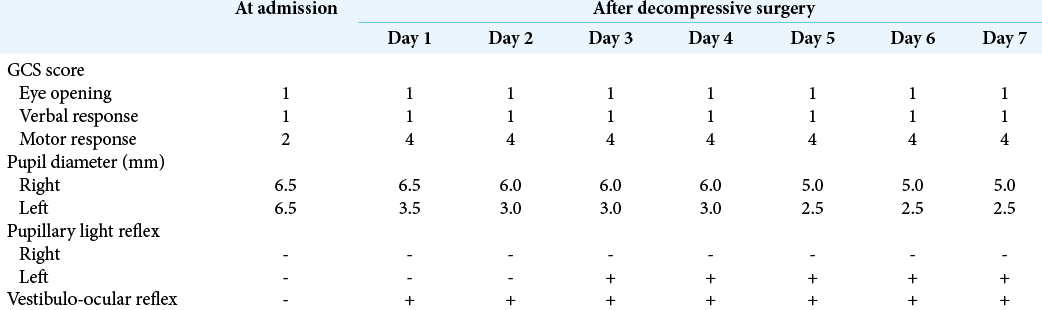
A 55-year-old man was found unconscious on the street and transferred to the emergency center of our hospital. At admission, the patient’s vital signs were stable, but he was unresponsive, the Glasgow Coma Scale (GCS) score was 4 (eye opening, 1; verbal response, 1; and motor response, 2), both pupils were maximally dilated (diameter, 6.5 mm), and pupillary light reflexes on both sides and vestibulo- ocular reflex (VOR) were absent. There were no visible local head injuries. Head CT revealed massive acute subdural hematoma above the right cerebral convexity causing prominent brain shift with subfalcine and transtentorial herniation, the obliteration of basal cisterns, as well as diffuse subarachnoid hemorrhage [
Figure 1:
Sequential head CT examinations in a 55-year-old man with severe traumatic brain injury. At the time of admission (a-c), massive acute subdural hematoma above the right cerebral convexity causing prominent brain shift with subfalcine and transtentorial herniation, the obliteration of basal cisterns, as well as diffuse subarachnoid hemorrhage were seen. Immediately after surgery directed at the evacuation of subdural hematoma, right temporal lobectomy, and external decompression (d-f), the “re-appeared” ambient cistern can be clearly visualized, as well as wide area of infarction within the right parietal and occipital lobes caused by compression of the posterior cerebral artery at the time of herniation, and subcutaneous hematoma. At the time of discharge after cranioplasty and ventriculoperitoneal shunting (g-i), asymmetric hydrocephalus, extensive infarction of the right parietal and occipital lobes, and small epidural CSF collection are evident, as well as absence of the right temporal muscle (arrows), which was resected at the time of decompressive surgery.
Postoperative course
Immediately after surgery, CT demonstrated significant reduction of the brain shift, “reappearance” of the ambient cistern, large area of infarction within the right parietal and occipital lobes caused by compression of the posterior cerebral artery at the time of herniation, and subcutaneous hematoma [
Table 1:
Dynamics of consciousness level and related reflexes in a reported patient with malignant cerebral swelling caused by severe traumatic brain injury with massive right-sided acute subdural hematoma causing prominent brain shift and subfalcine and transtentorial herniation, who underwent craniectomy with aggressive internal and external decompression.
DISCUSSION
It is well recognized that decompressive craniectomy may be quite effective for ICP control in cases of malignant cerebral swelling caused by massive acute stroke and severe traumatic brain injury.[
Internal decompression with temporal lobectomy also demonstrated its usefulness in control of ICP in cases of malignant cerebral swelling caused either by massive hemispheric infarction or by severe traumatic brain injury.[
Swelling of the temporal muscle in the presence of bone defect after decompressive craniectomy may result in additional “secondary” brain compression. For avoidance of such a complication, extensive resection of the temporal muscle and fascia down to the zygomatic arch has been proposed.[
Finally, any decompressive craniectomy should presume wide lax duraplasty to cover the entire bone defect, which can be done with the use of various materials.[
Decompressive craniectomy is usually performed as life- saving surgery for neurosurgical emergencies, such as massive cerebral infarction or severe traumatic brain injury. In these cases, it may be quite difficult to predict subsequent clinical course, in particular, the possible development of uncontrolled intracranial hypertension with secondary brainstem compression. Therefore, if such surgery is performed in deeply comatose patient with transtentorial herniation and intraoperative evidence of malignant cerebral swelling, for achievement of the maximum effects on the ICP control, combination of the various techniques for internal and external decompression of the brain may be reasonable. As demonstrated in the presented case, such an aggressive surgical strategy may be beneficial for survival, while further experience is needed for detailed evaluation of the long-term functional outcomes.
CONCLUSION
Aggressive internal and external decompression with combined use of large size craniectomy, extensive temporal lobectomy, removal of the bulk of the temporal muscle and fascia down to the zygomatic arch, and wide lax duraplasty should be considered as possible life-saving surgical option in cases of neurosurgical emergencies accompanied by malignant cerebral swelling, prominent brain shift, and transtentorial herniation.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Gibbs CH, Mahan PE, Lundeen HC, Brehnan K, Walsh EK, Sinkewiz SL. Occlusal forces during chewing-influences of biting strength and food consistency. J Prosthet Dent. 1981. 46: 561-7
2. Hakan AK, Daltaban IS, Vural S. The role of temporal lobectomy as a part of surgical resuscitation in patients with severe traumatic brain injury. Asian J Neurosurg. 2019. 14: 436-9
3. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]: A multicentre, open, randomised trial. Lancet Neurol. 2009. 8: 326-33
4. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): A randomized, controlled trial. Stroke. 2007. 38: 2518-25
5. Kapapa T, Brand C, Wirtz CR, Woischneck D. Outcome after decompressive craniectomy in different pathologies. World Neurosurg. 2016. 93: 389-97
6. Lanzino G. Decompressive craniectomy for acute stroke: Early is better. J Neurosurg. 2008. 109: 285-6
7. Nussbaum ES, Wolf AL, Sebring L, Mirvis S. Complete temporal lobectomy for surgical resuscitation of patients with transtentorial herniation secondary to unilateral hemispheric swelling. Neurosurgery. 1991. 29: 62-6
8. Park J, Kim E, Kim GJ, Hur YK, Guthikonda M. External decompressive craniectomy including resection of temporal muscle and fascia in malignant hemispheric infarction. J Neurosurg. 2009. 110: 101-5
9. Quinn TM, Taylor JJ, Magarik JA, Vought E, Kindy MS, Ellegala DB. Decompressive craniectomy: Technical note. Acta Neurol Scand. 2011. 123: 239-44
10. Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004. 55: 55-62
11. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007. 38: 2506-17
12. Winter CD, Adamides A, Rosenfeld JV. The role of decompressive craniectomy in the management of traumatic brain injury: A critical review. J Clin Neurosci. 2005. 12: 619-23
13. Yu SH, Kim BC, Choi JY, Lee JI, Cho WH, Choi HJ. Addition of resection of temporal muscle and fascia in decompressive craniectomy in the treatment of traumatic brain injury. Korean J Neurotrauma. 2016. 12: 84-8