- Department of Neurosurgery, Kocaeli University, School of Medicine, Kocaeli, Turkey.
- Department of Pathology, Kocaeli University, School of Medicine, Kocaeli, Turkey.
Correspondence Address:
Harun Emre Sen, Department of Neurosurgery, Kocaeli University, School of Medicine, Kocaeli, Turkey.
DOI:10.25259/SNI_854_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Harun Emre Sen1, Busra Yaprak Bayrak2, Volkan Etus1. Aggressive osteoblastoma of the cervical spine and resultant complication due to swollen oxidized regenerated cellulose: A case report. 19-Jan-2024;15:20
How to cite this URL: Harun Emre Sen1, Busra Yaprak Bayrak2, Volkan Etus1. Aggressive osteoblastoma of the cervical spine and resultant complication due to swollen oxidized regenerated cellulose: A case report. 19-Jan-2024;15:20. Available from: https://surgicalneurologyint.com/surgicalint-articles/12711/
Abstract
Background: Osteoblastomas, although rare, are benign primary bone tumors, with cervical spine involvement being exceptionally uncommon. Late diagnosis, especially in aggressive cases, can lead to surgical challenges. Oxidized regenerated cellulose (ORC) used for hemostasis may result in complications if left in the surgical field.
Case Description: An 8-year-old female presented with six months of intractable neck pain accompanied by swelling, hindering proximal right upper extremity evaluation. Motor strength was intact distally, with normal reflexes and no hypoesthesia. Imaging revealed a C4–5 facet joint lesion necessitating surgery. Intraoperative hemorrhage prompted ORC application, which led to postoperative arm pain and C5–6 radiculopathy. Subsequent surgery alleviated these symptoms.
Conclusion: Osteoblastomas, despite their benign classification, may exhibit aggressive characteristics, warranting en-bloc resection. Cervical spine osteoblastomas, due to their vascular nature and proximity to vital structures, complicate surgical interventions. ORC, a commonly used hemostatic agent, may induce compression complications, and early intervention is critical for patient recovery. This case underscores the intricacies of managing aggressive osteoblastomas in the cervical spine and highlights potential ORC-related complications. Surgeons must exercise caution when using ORC and consider postoperative risks. Prompt intervention and meticulous planning are paramount for favorable outcomes in such cases.
Keywords: Cervical spine osteoblastoma, Complication, Swollen oxidized regenerated cellulose
INTRODUCTION
Osteoblastomas are rare and benign primary bone tumors that account for 1% of all primary bone tumors. About 40% of them occur in the spine, predominantly involving the posterior elements.[
Although complete removal of ORC after hemostasis is recommended, leaving a certain amount in place is often preferred to prevent postoperative hematoma.[
CASE
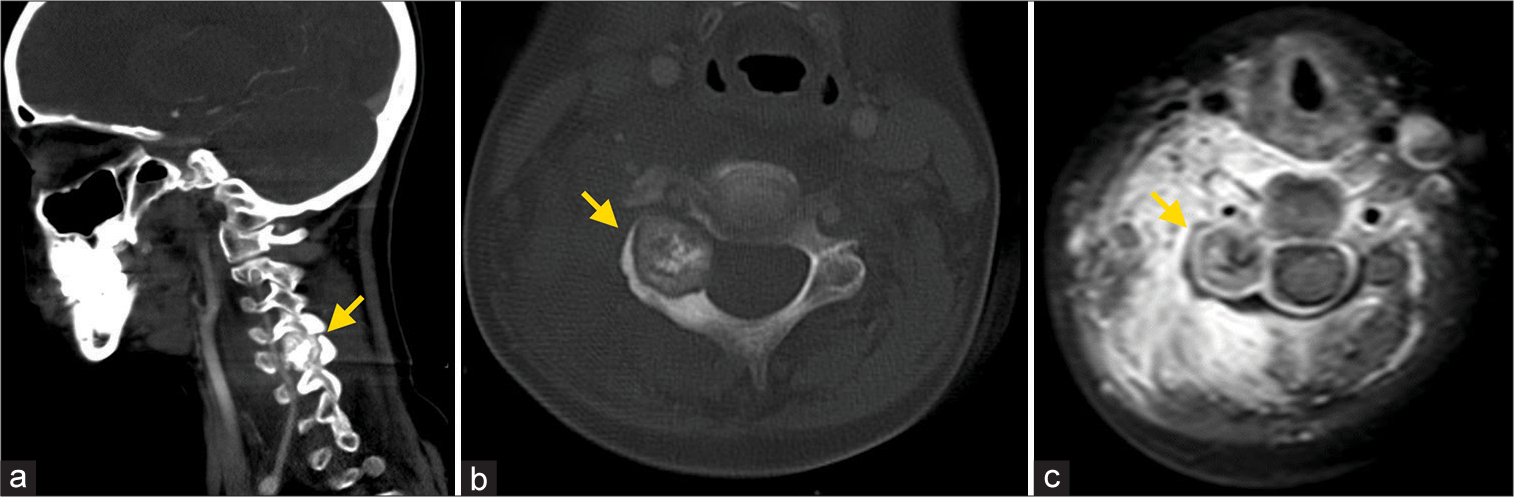
An 8-year-old female patient presented with a 6-month history of progressively worsening neck pain and tenderness, unresponsive to anti-inflammatory medication. Physical examination revealed swelling in the right posterior neck and increased pain during shoulder movements and palpation. Due to pain, a comprehensive assessment of proximal movements of the right upper extremity could not be performed, but normal motor strength was observed distally. Deep tendon reflexes were bilaterally normal, and no hypoesthesia was detected. A computed tomography (CT) scan revealed a 21 mm lesion at the level of the C4–5 right pedicle and articular facet, extending to the lamina and showing minimal protrusion into the spinal canal. CT angiography imaging confirmed that the lesion was not associated with the vertebral artery. Gadolinium-enhanced magnetic resonance imaging (MRI) demonstrated a contrast-enhancing mass with ossification signals in the central and outer matrix [
Figure 1:
(a) Sagittal angio-CT scan indicate the presence of a tumor (arrow) at the level of the C4-5 right pedicle and articular facet. (b) The inhomogeneous matrix ossification is presented in the tumor (arrow). (c) Gadolinium-enhanced axial MRI depicted a well-demarcated tumor (arrow) and edema of the paravertebral soft tissue.
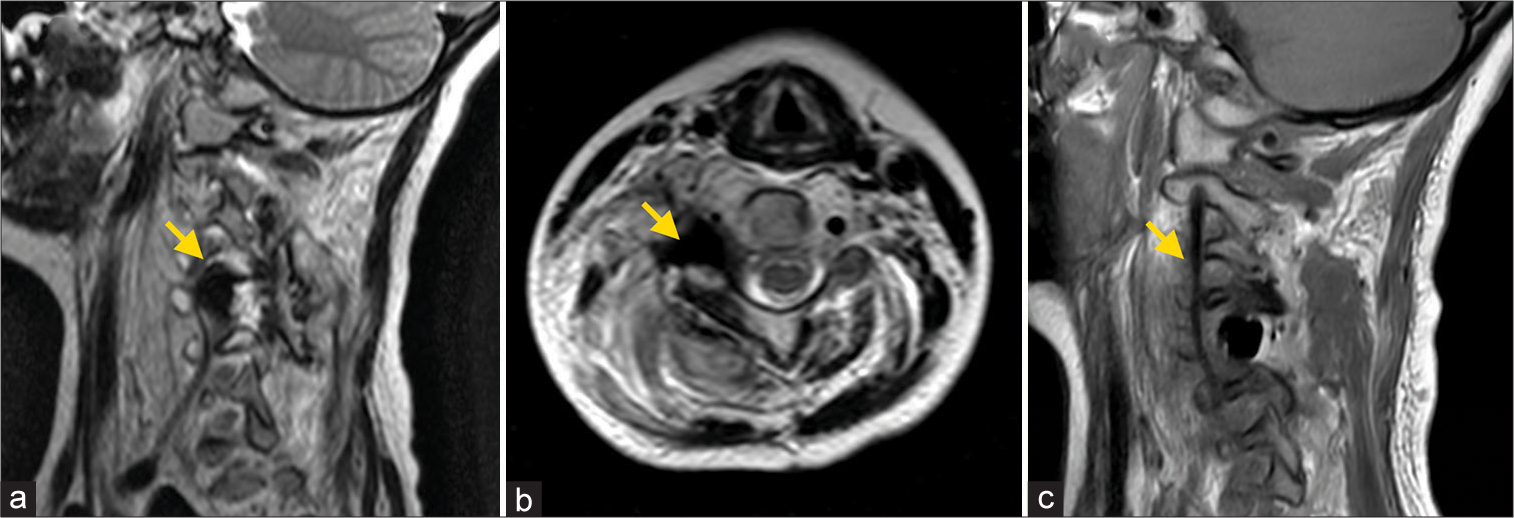
The tumor was removed in fragments, with a significant hemorrhage encountered during the excision of the final portion adjacent to the vertebral artery. We were aware that bleeding might be encountered during excision due to the high vascularity of the tumor. However, the bleeding flow rate exceeded our estimation (approximately 250 cc until hemostasis), which intraoperatively prevented us from distinguishing whether the cause of bleeding was of vertebral artery origin. Hemostasis was promptly achieved through the application of ORC compression. No additional hemostatic agent was applied. To mitigate the risk of postoperative hemorrhage recurrence, a portion of the ORC was intentionally retained in the surgical area. Following surgery, the patient experienced severe arm pain and exhibited grade 2/5 muscle strength in arm abduction and elbow flexion, indicative of C5–6 radiculopathy. Imaging studies revealed no damage to the vertebral artery but identified a mass within the excised tumor bed compressing the C5 nerve root, displaying hypointensity on T2-weighted MRIs [
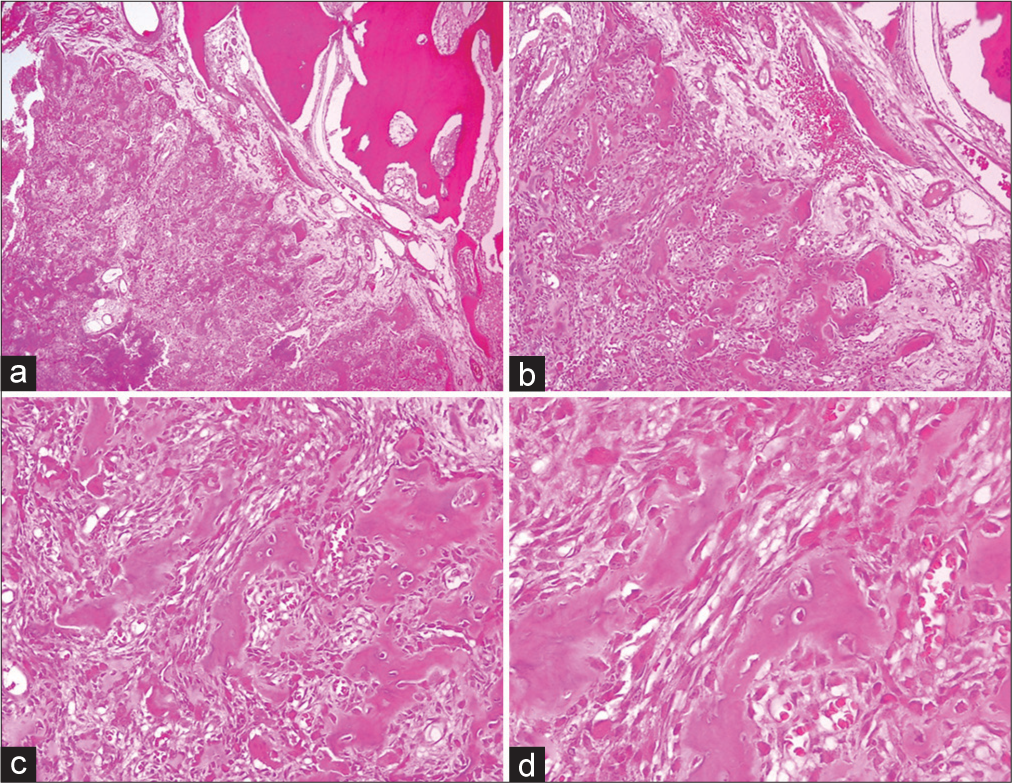
Consequently, the patient underwent a second surgery for exploration and decompression the following day. During the second operation, no hematoma was observed, but the previously retained ORC had expanded due to swelling. Most of the ORC was removed to alleviate compression on the dura and nerve root, with a minimal amount left in the area where hemorrhage had occurred. Subsequently, the patient’s severe arm pain subsided, and muscle strength improved to an early-stage grade of 3/5 for the deltoid, biceps, and brachioradialis. Following eight months of physiotherapy, the patient regained a muscle strength grade of 5/5. Pathological examination of the surgical specimen confirmed the presence of osteoblastoma [
Figure 3:
(a) Expansile tumor surrounded by a sclerotic rim, showing a spectrum of bone maturation changes (H&E, ×40). (b) Neoplasia with diffusely distributed osteoclast-type multinucleated giant cells with extravasated erythrocytes (H&E, ×100). (c) Bone trabeculae are covered by a single layer of osteoblasts (H&E, ×200). (d) Benign bone tumor with degenerative cytological atypia characterized by cells with large degenerated nuclei and smudged chromatin (H&E, ×400).
DISCUSSION
Although classified as a benign tumor, osteoblastoma shows high vascularity and can exhibit locally aggressive growth. Typically, it involves the posterior elements of the spine.[
ORC is often preferred in neurosurgery to achieve hemostasis. Although it is a biologically absorbable material, it is recommended to remove ORC altogether after achieving hemostasis.[
CONCLUSION
We presented a case of an 8-year-old female patient with aggressive osteoblastoma involving the posterior elements of the cervical vertebra. These benign tumors, which have relatively high rates of malignant transformation and recurrence, ideally require en bloc total resection. However, due to their highly vascular nature, surgery can be challenging. Although it is a reliable hemostatic agent, it should not be preferred to leave ORC in the vicinity of nerve roots and the spinal canal. When planning to leave the ORC in the surgical area, keeping its presence to a minimum is crucial. Otherwise, it may lead to unexpected complications through mass effect, necessitating a second surgical intervention.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to note that there were no external sources of assistance or funding for this case report. All aspects of this research were conducted within our medical institution.
References
1. Ando K, Imagama S, Kobayashi K, Nishida Y, Ishiguro N. Aggressive osteoblastoma of the cervical spine involving the canal and vertebral artery: A case report. Eur Spine J. 2017. 26: 111-6
2. Boriani S, Amendola L, Bandiera S, Simoes CE, Alberghini M, Di Fiore M. Staging and treatment of osteoblastoma in the mobile spine: A review of 51 cases. Eur Spine J. 2012. 21: 2003-10
3. Brodbelt AR, Miles JB, Foy PM, Broome JC. Intraspinal oxidised cellulose (Surgicel) causing delayed paraplegia after thoracotomy--a report of three cases. Ann R Coll Surg Engl. 2002. 84: 97-9
4. Dogan S, Kocaeli H, Doygun M. Oxidised regenerated cellulose as a cause of paraplegia after thoracotomy: Case report and review of the literature. Spinal Cord. 2005. 43: 445-7
5. Feng G, Huang K, Li L, Gong Q, Liu H, Song Y. Treatment of osteoblastoma at C3-4 in a child: A case report. BMC Musculoskelet Disord. 2014. 15: 313
6. Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive “benign” primary spine neoplasms: Osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine (Phila Pa 1976). 2009. 34: S39-47
7. Henry MC, Tashjian DB, Kasowski H, Duncan C, Moss RL. Postoperative paraplegia secondary to the use of oxidized cellulose (Surgicel). J Pediatr Surg. 2005. 40: E9-11
8. Kanakis MA, Chatzis A, Papadopoulos E, Contrafouris C, Azariades P, Karabinis A. Post thoracotomy spinal cord compression in a child. A word of caution. Int J Surg Case Rep. 2013. 4: 354-6
9. Kaner T, Sasani M, Oktenoglu T, Aydin S, Ozer AF. Osteoid osteoma and osteoblastoma of the cervical spine: The cause of unusual persistent neck pain. Pain Physician. 2010. 13: 549-54
10. Lian X, Zhao J, Hou T, Ma H, Chen Z. Benign intraspinal osteoblastoma stemming from C7 lamina in cervicothoracic junction: A case report. Spine (Phila Pa 1976). 2006. 31: E895-9
11. Menovsky T, Plazier M, Rasschaert R, Maas AI, Parizel PM, Verbeke S. Massive swelling of Surgicel® Fibrillar™ hemostat after spinal surgery. Case report and a review of the literature. Minim Invasive Neurosurg. 2011. 54: 257-9
12. Oliveira C, Vital L, Serdoura F, Pinho AR, Veludo V. Cervical osteoblastoma: A case report. Rev Bras Ortop (Sao Paulo). 2019. 54: 219-22
13. Oto A, Remer EM, O’Malley CM, Tkach JA, Gill IS. MR characteristics of oxidized cellulose (Surgicel). AJR Am J Roentgenol. 1999. 172: 1481-4
14. Ozaki T, Liljenqvist U, Hillmann A, Halm H, Lindner N, Gosheger G. Osteoid osteoma and osteoblastoma of the spine: Experiences with 22 patients. Clin Orthop Relat Res. 2002. 397: 394-402
15. Samdani A, Torre-Healy A, Chou D, Cahill AM, Storm PB. Treatment of osteoblastoma at C7: A multidisciplinary approach. A case report and review of the literature. Eur Spine J. 2009. 18: 196-200
16. Saglik Y, Atalar H, Yildiz Y, Basarir K, Gunay C. Surgical treatment of osteoblastoma: A report of 20 cases. Acta Orthop Belg. 2007. 73: 747-53
17. Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004. 13: S89-96
18. Zileli M, Cagli S, Basdemir G, Ersahin Y. Osteoid osteomas and osteoblastomas of the spine. Neurosurg Focus. 2003. 15: E5