- Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Ramesh S. Doddamani
Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India
DOI:10.4103/sni.sni_328_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Ramesh S. Doddamani, Rajesh K. Meena, Dattaraj Sawarkar. Ambiguity in the Dural Tail Sign on MRI. 19-Mar-2018;9:62
How to cite this URL: Ramesh S. Doddamani, Rajesh K. Meena, Dattaraj Sawarkar. Ambiguity in the Dural Tail Sign on MRI. 19-Mar-2018;9:62. Available from: http://surgicalneurologyint.com/surgicalint-articles/ambiguity-in-the-dural-tail-sign-on-mri/
Abstract
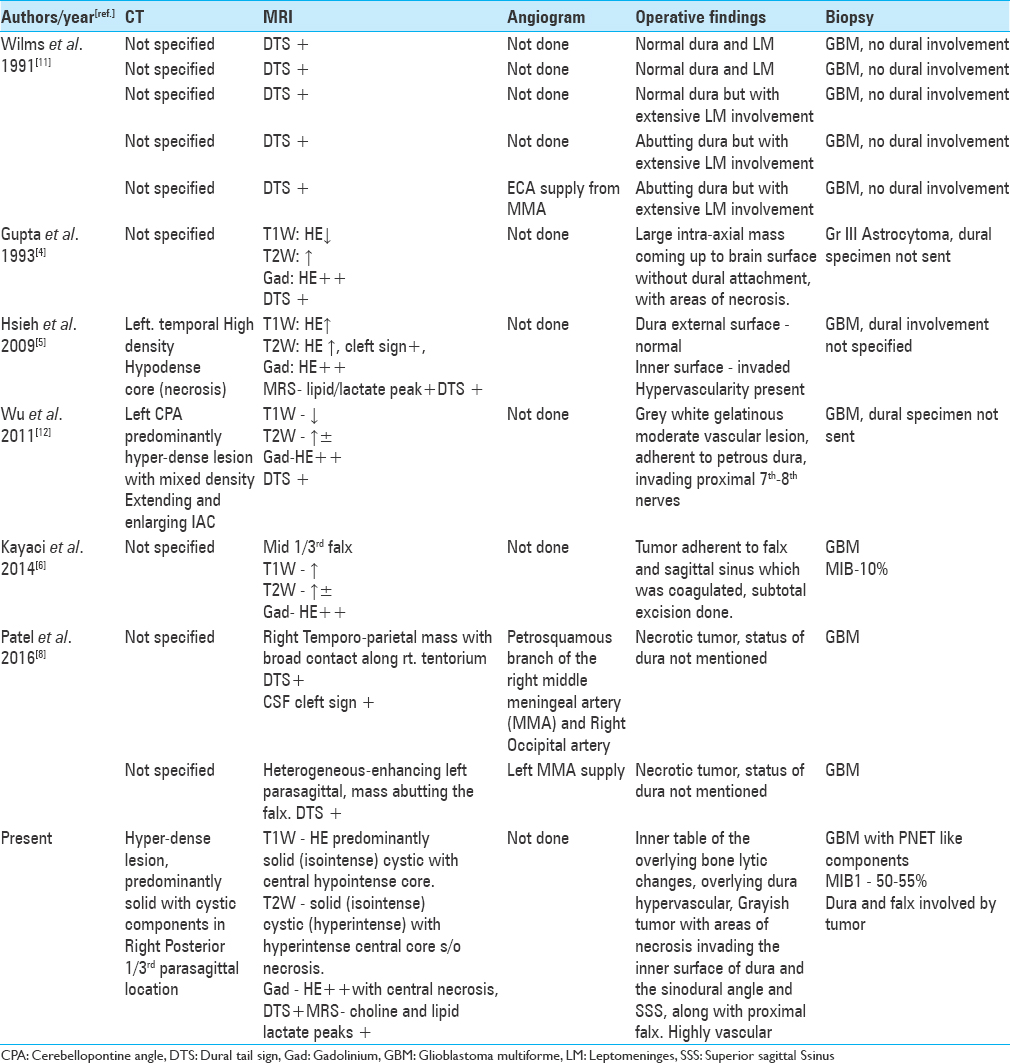
Background:Meningiomas give rise to the dural tail sign (DTS) on contrast-enhanced magnetic resonance imaging (CEMRI). The presence of DTS does not always qualify for a meningioma, as it is seen in only 60-72% of cases. This sign has been described in various other lesions like lymphomas, metastasis, hemangiopericytomas, schwannomas and very rarely glioblastoma multiforme (GBM). The characteristics of dural-based GBMs are discussed here, as only eleven such cases are reported in the literature till date. Here we discuss the unique features of this rare presentation.
Case Description:A 17-year-old male presented to the emergency department (ED) with, complaints of headache, recurrent vomiting, vision loss in right eye and altered sensorium. On examination patient was drowsy with right hemiparesis, secondary optic atrophy in the right eye and papilledema in the left eye. MRI brain showed, heterogeneous predominantly solid cystic lesion with central hypo-intense core suggestive of necrosis with heterogeneous enhancement and a positive DTS. Patient underwent emergency left parasagittal parieto-occipital craniotomy and gross total tumor excision including the involved dura and the falx. On opening the dura, tumor was surfacing, invading the superior sagittal sinus and the falx, greyish, soft to firm in consistency with central necrosis and highly vascular suggesting a high-grade lesion. Postoperative computed tomography (CT) of the brain showed evidence of gross total tumor (GTR) excision. The postoperative course of the patient was uneventful. Histopathological analysis revealed GBM with PNET like components. The dura as well as the falx were involved by the tumor.
Conclusion:GBMs can arise in typical locations along with DTS mimicking meningiomas. Excision of the involved dura and the falx becomes important in this scenario, so as to achieve GTR. Hence high index of suspicion preoperatively aided by Magnetic Resonance Imaging (MRS) can help distinguish GBMs from meningioma, thereby impacting upon the prognosis.
Keywords: Dural tail sign, glioblastoma multiforme, meningioma, posterior third parasagittal
INTRODUCTION
Dural tail sign (DTS) is considered the hallmark for the radiological diagnosis of a meningioma. It is seen in 60–72% cases of meningiomas and would represent either direct tumor invasion or reactive changes surrounding the tumor itself.[
CASE REPORT
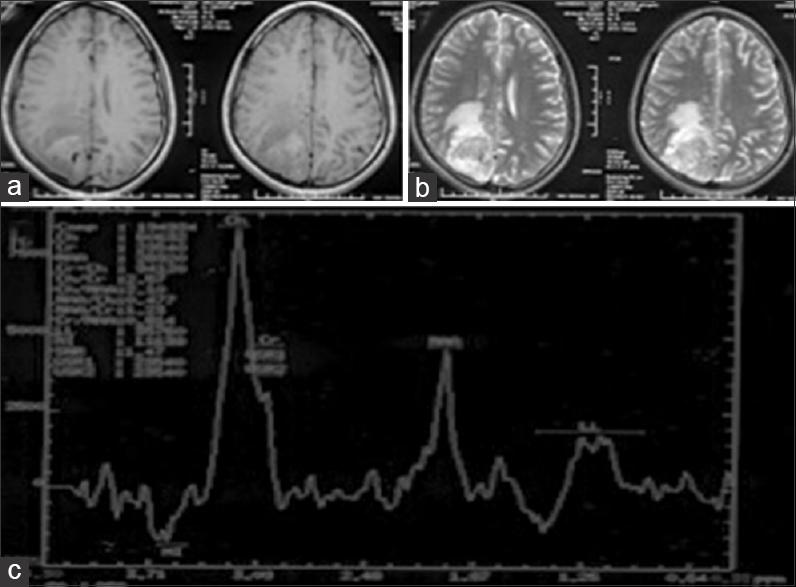
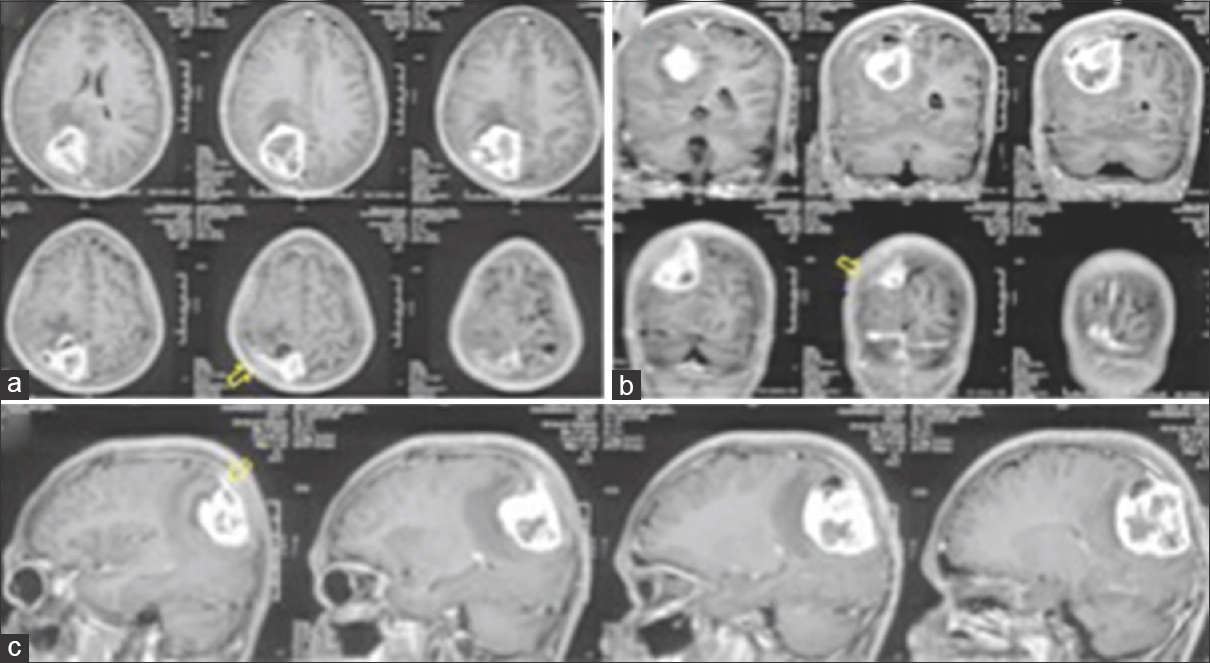
A 17-year-old male with no comorbidities presented to the emergency department (ED) with complaints of headache and recurrent vomiting for 2 weeks, vision loss in right eye for 1 week, and altered sensorium for 2 days. On examination, the patient was drowsy but arousable, right hemiparesis grade 4/5, right-sided secondary optic atrophy, and left-sided papilledema (pseudofoster Kennedy syndrome). Magnetic resonance imaging (MRI) of the brain showed, T1-weighted images heterogeneous predominantly solid (iso-intense) cystic with central hypo-intense core suggestive of necrosis [
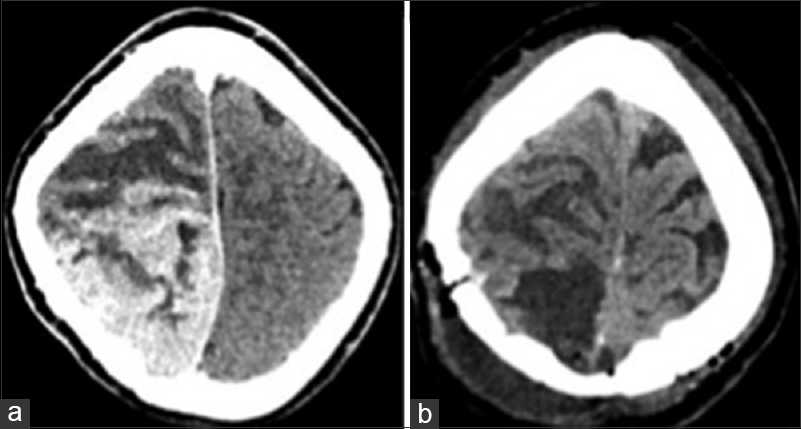
Patient was taken up for emergency surgery, and left parasagittal parieto-occipital craniotomy fashioned and gross total tumor excision was done. On opening the dura, tumor was seen surfacing and invading the superior sagittal sinus as well as the falx, with infiltration into the adjacent brain parenchyma. Tumor was greyish soft to firm in consistency with central necrosis and highly vascular suggestive of a high-grade lesion. Per-operatively patient had a transient episode of hypotension, which was managed. Approximate blood loss was 2 liters. Postoperative computed tomography scans showed complete tumor removal [
DISCUSSION
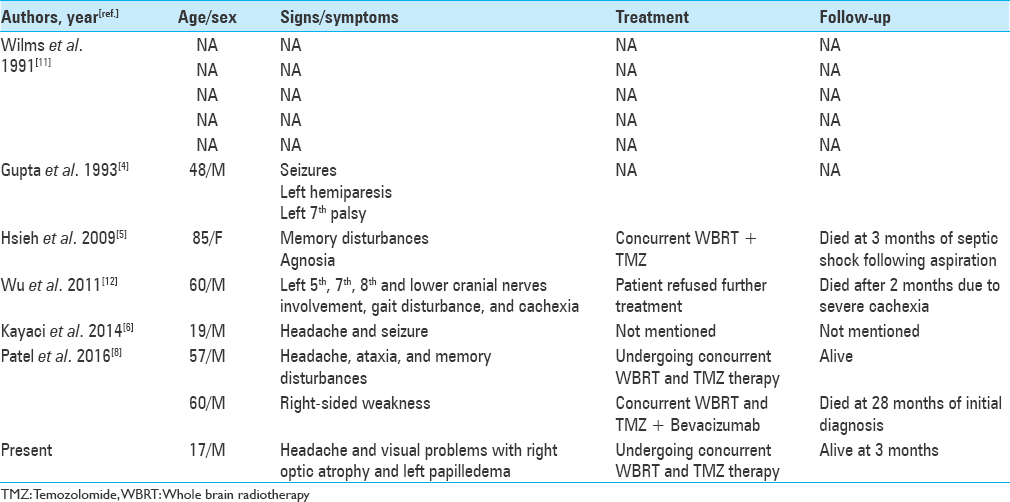
The presence of dural tail sign on MRI is highly suggestive of a meningioma but not a pathognomonic sign. The presence of a dural tail in GBM is very rare, and a thorough review of English literature revealed 10 cases of GBM exhibiting DTS mimicking meningioma. The demographic and clinical data are listed in
Linear enhancement was present along the duramater originating from and extending outward from the tumor margin Enhancement was greater than elsewhere along the dura Findings were present in the two different imaging planes There was agreement among three observers.
All the criteria were fulfilled in our case, thereby confirming DTS leading to the provisional diagnosis of a meningioma.
Wilms et al.[
Unlike meningiomas, GBMs are highly vascular and aggressive lesions invading normal brain, deriving their blood supply from pial vasculature. The vessels of duramater rarely feed GBMs, the enhanced dural tail sign is likely to develop from vascular congestion or proliferation.[
Magnetic resonance spectroscopy (MRS) is a useful adjunct in differentiating preoperatively meningiomas from GBMs. Majos et al.[
The origin of extra-axial GBMs has been a matter of debate. Various authors have proposed two mechanisms by which these lesions develop. One hypothesis involving GBM of the cranial nerves (CN) states that, GBM arise primarily from the CNS tissue that lay within the proximal parts of the CN itself. CNS tissue may extend well into the CN, and isolated islands of CNS tissue may even be found within the CN at a considerable distance from its exit point. The second hypothesis is that the tumor originated as primary in the heterotopic neuroglial cell nests in the leptomeninges of the adjacent brain.[
CONCLUSION
Lesions arising in typical locations for meningiomas but with atypical appearances, GBM should be considered in the differential diagnosis. MRS is a very valuable method of differentiating GBMs from meningiomas. Preoperative angiography appears to have a role in reducing the blood loss although not performed in the present case. High index of suspicion prior to surgery and excision of the involved dural elements would lead to a better outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aoki S, Sasaki Y, Machida T, Tanioka H. Contrast-enhanced MR images in patients with meningioma: Importance of enhancement of the dura adjacent to the tumor. AJNR Am J Neuroradiol. 1990. 11: 935-8
2. Goldsher D, Litt AW, Pinto RS, Bannon KR, Kricheff II. Dural “tail”associated with meningiomas on Gd-DTPA-enhanced MR images: Characteristics, differential diagnostic value, and possible implications for treatment. Radiology. 1990. 176: 447-50
3. Guermazi A, Lafitte F, Miaux Y, Adem C, Bonneville JF, Chiras J. The dural tail sign beyond meningioma. Clin Radiol. 2005. 60: 171-88
4. Gupta S, Gupta RK, Banerjee D, Gujral RB. Problems with the “dural tail” sign. Neuroradiology. 1993. 35: 541-2
5. Hsieh CT, Liu MY, Tang CT, Sun JM, Tsai WC, Hsia CC. Problem of dural tail sign in glioblastoma multiforme?. Acta Neurol Belg. 2009. 109: 310-3
6. Kayaci S, Şengöz A, Köksal V, Gunver F, Kiliç K. Glioblastoma multiforme mimicking falx meningioma with achondroplasia. Neurosurgery Quarterly. 2014. 24: 53-5
7. Majos C, Alonso J, Aguilera C, Serrallonga M, Perez- Martin J. Proton magnetic resonance spectroscopy ((1) H MRS) of human brain tumours: Assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol. 2003. 13: 582-91
8. Patel M, Nguyen HS, Doan N, Gelsomino M, Shabani S, Mueller W. Glioblastoma Mimicking Meningioma: Report of 2 Cases. World Neurosurg 2016;95:624. e-. 4. p.
9. Reifenberger G, Boström J, Bettag M, Bock WJ, Wechsler W, Kepes J. Primary glioblastoma multiforme of the oculomotor nerve.Case report. J Neurosurg. 1996. 84: 1062-6
10. Tokumaru A, O'uchi T, Eguchi T, Kawamoti S, Kokubo T, Suzuki M. Prominent meningeal enhancement adjacent to meningioma on Gd-DTPA-enhanced MR images: Histopathologic correlation. Radiology. 1990. 175: 431-3
11. Wilms G, Lammens M, Marchal G, Van Calenbergh F, Plets C, Van Fraeyenhoven L. Thickening of dura surrounding meningiomas: MR features. J Comput Assist Tomogr. 1989. 13: 763-8
12. Wu B, Liu W, Zhu H, Feng H, Liu J. Primary glioblastoma of the cerebellopontine angle in adults. J Neurosurg. 2011. 114: 1288-93