- Department of Trauma and Acute Care Surgery, Kettering Health Main Campus, Kettering, Ohio, United States
- Department of Radiology, Kettering Health Main Campus, Kettering, Ohio, United States
- Department of Kettering Brain and Spine, Kettering Health Main Campus, Kettering, Ohio, United States.
Correspondence Address:
Brandi Palmer, Department of Trauma and Acute Care Surgery, Kettering Health Main Campus, Kettering, Ohio, United States.
DOI:10.25259/SNI_607_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brandi Palmer1, Melody Campbell1, Kellie Maertz1, Laurie Narigon1, Karen Herzing1, Heena Santry1, William Boyce2, Ragavan Narayanan1, Akil Patel3. Analysis of middle meningeal artery embolization for the treatment of chronic, acute on chronic, and subacute subdural hematomas. 01-Mar-2024;15:71
How to cite this URL: Brandi Palmer1, Melody Campbell1, Kellie Maertz1, Laurie Narigon1, Karen Herzing1, Heena Santry1, William Boyce2, Ragavan Narayanan1, Akil Patel3. Analysis of middle meningeal artery embolization for the treatment of chronic, acute on chronic, and subacute subdural hematomas. 01-Mar-2024;15:71. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12772
Abstract
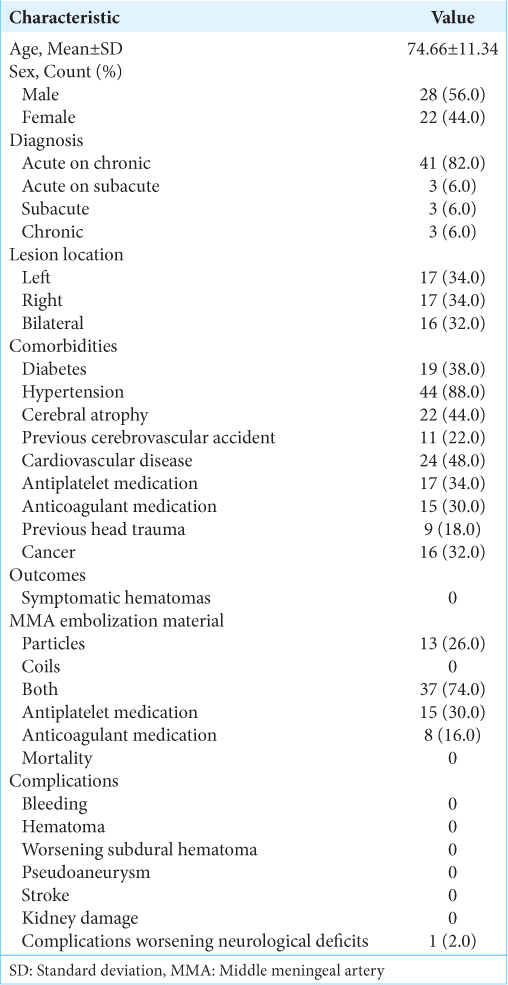
Background: Chronic subdural hematoma (cSDH) is a common sequela of traumatic brain injury. Middle meningeal artery embolization (MMAE) has shown promising results as an emerging minimally invasive alternative treatment. The purpose of this study is to examine the safety and efficacy of MMAE performed in patients with cSDH, acute-on-chronic, and subacute SDH with a traumatic etiology.
Methods: This retrospective study included cases performed at a Level II Trauma Center between January 2019 and December 2020 for MMAE of cSDHs. Data collected included patient demographic characteristics and comorbidities, SDH characteristics, complications, and efficacy outcomes. The lesion measurements were collected before the procedure, 4–6 weeks and 3–6 months post-procedure.
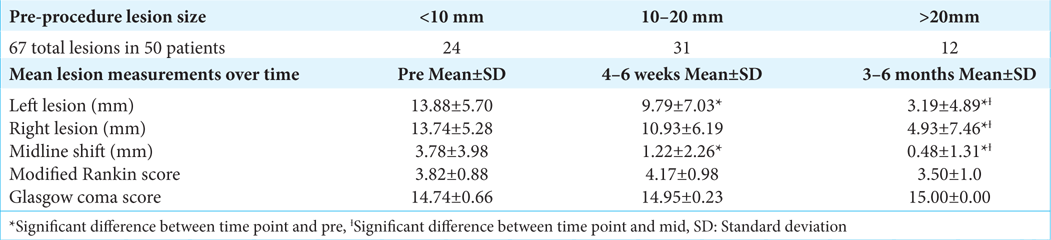
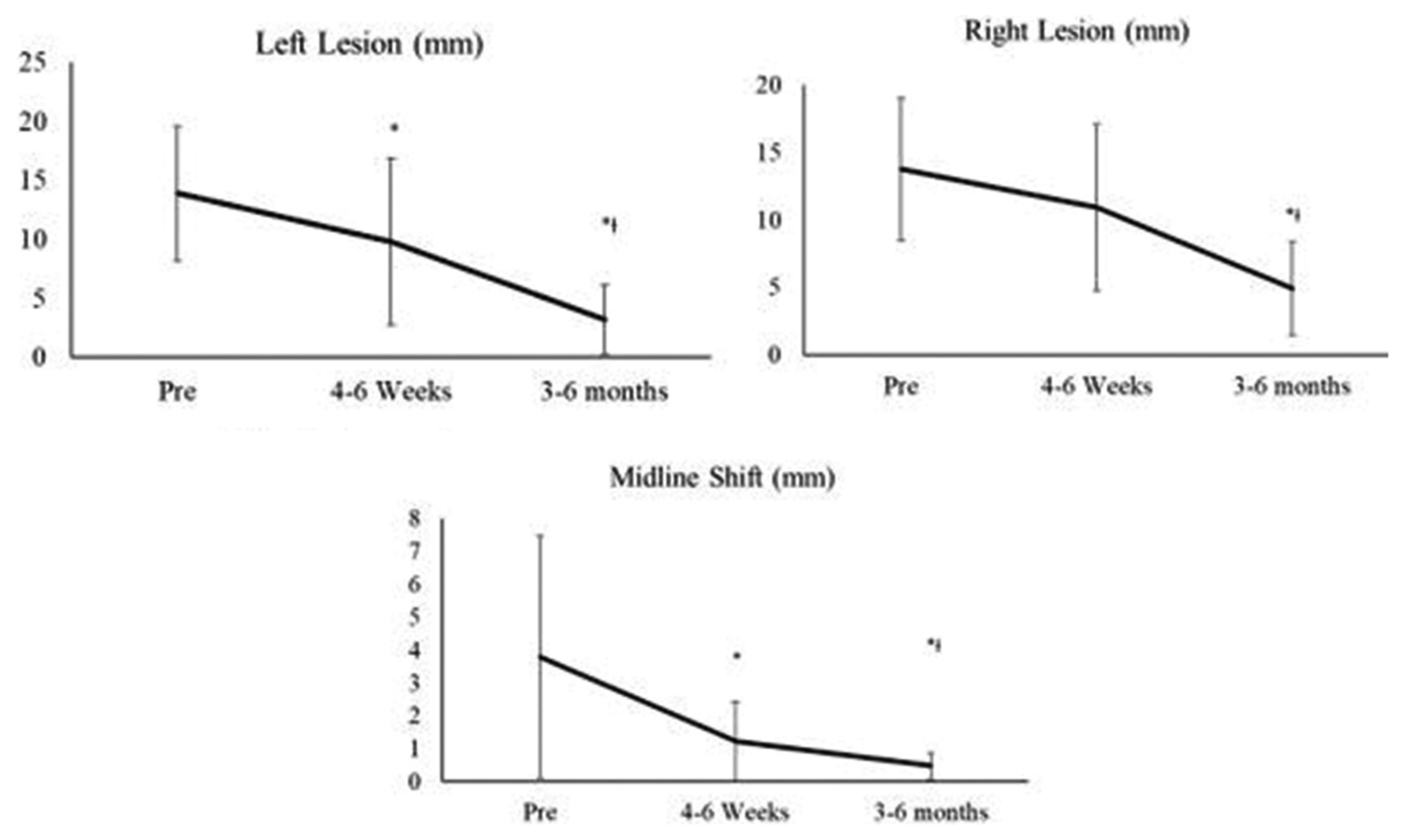
Results: In our patient population, 78% (39) either had lesions improve or completely resolved. The sample included 50 patients with a mean age of 74 years old. Statistically significant reductions in lesion size were found from pre- to post-procedure in the left lesions, right lesions, and midline shifts. The left lesions decreased from 13.88 ± 5.70 mm to 3.19 ± 4.89 mm at 3–6 months with P P = 0.02. Midline shifts decreased from 3.78 ± 3.98 mm to 0.48 ± 1.31 mm at 3–6 months with P = 0.02. No complications were experienced for bleeding, hematoma, worsening SDH, pseudoaneurysm, or stroke.
Conclusion: Our pilot study from a single center utilizing MMAE demonstrates that MMAE is successful without increasing treatment-related complications not only for cSDH but also in acute-on-cSDH and SDH with a subacute component.
Keywords: Interventional radiology, Middle meningeal artery, Neurosurgery, Subdural hematoma, Trauma
INTRODUCTION
Chronic subdural hematoma (cSDH) is an increasingly common sequela of traumatic brain injury. It is estimated that by 2030, there will be 60,000 new cases of cSDH each year, largely due to an increase in the elderly population and widespread use of anticoagulation and antiplatelet medications. This includes those with a high risk of ground-level falls due to frailty and instability due to other chronic diseases.[
Burr-hole irrigation surgery has traditionally been considered the gold standard treatment for cSDH requiring operative management.[
A cSDH is often precipitated by relatively mild head trauma, particularly in the elderly population. This results in an acute subdural hematoma (SDH), which may not be initially significant enough to cause neurologic deficit or hospitalization.[
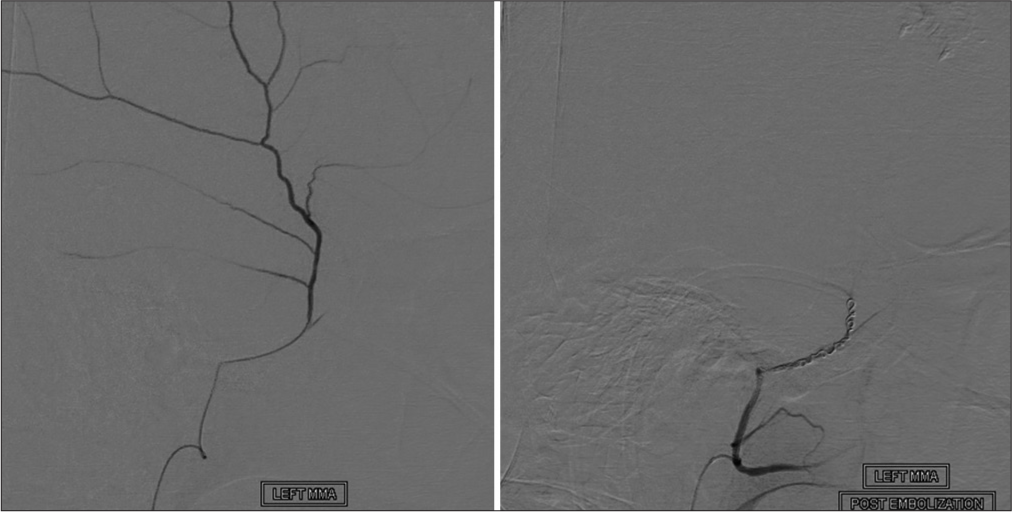
Over the past several years, MMA embolization (MMAE) has shown promising results as an emerging minimally invasive alternative treatment for patients with cSDH. [
Despite its increase in utilization, publications have been largely limited to analyses of the procedure in small studies, and case reports focusing on cSDH and typically on patients with a non-traumatic etiology. The purpose of this single-site study is to retrospectively examine the safety and efficacy of MMAE performed in patients with not only cSDH but also acute-on-chronic and subacute SDH with a traumatic etiology. Most of the study cohort sustained traumatic injuries, with falls being the predominant mechanism of injury. In a review of studies since 2000, this patient population is drastically understudied. This analysis sought to add to the existing literature, hoping to benefit trauma and neurosurgery patients with a potential change in trauma practice.
MATERIALS AND METHODS
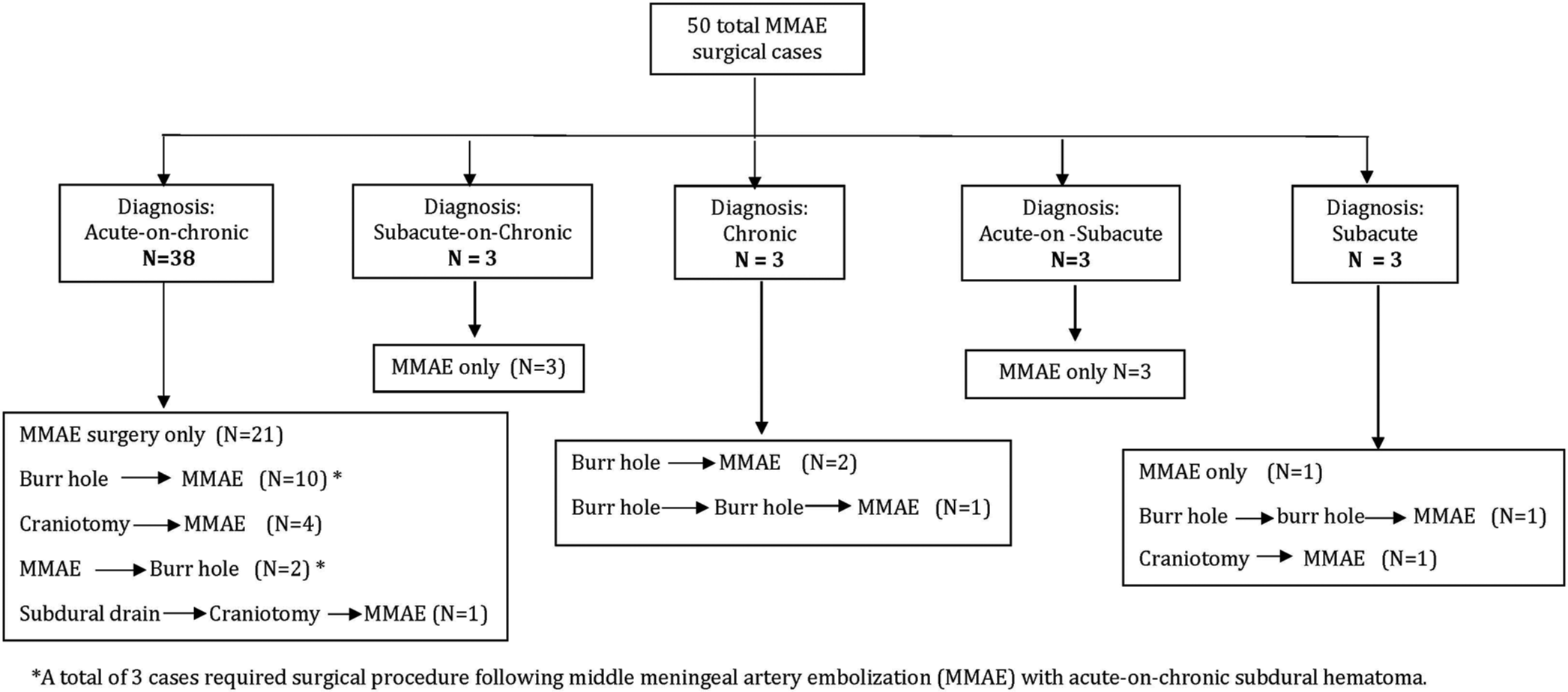
The study protocol was reviewed and approved by the Institutional Review Board. This single-center retrospective observational study included patients who were above the age of 18 and admitted to this Level II trauma center, and underwent MMAE between January 2019 and December 2020. Electronic medical records were utilized to obtain data. The study sample criteria included patients with computed tomography (CT) images confirming cSDH, acute-on-cSDH, acute-on-subacute, or subacute SDH with mass effect or midline shift while maintaining a Glasgow Coma Scale (GCS) of >13. The presence of a cSDH membrane was not necessary for study inclusion due to the variability of its presence on a CT image. Rather, patients experiencing symptoms of inflammation and bleeding provided indications for the MMAE procedure. Patients were excluded if there was a need for urgent intervention or if no subacute or chronic subdural component was identified.
Data collected included demographic characteristics and comorbidities, SDH characteristics, procedural details, complications, follow-up neurological examinations, and safety and efficacy outcomes. MMAE was performed by our neurosurgeons utilizing particles alone, coils, or a combination. SDH characteristics were determined by reports of CT of the head (non-contrast) as interpreted by a fellowship-trained neuroradiologist. Patient charts and images were examined for up to 6 months post-procedure.
The size of the SDH by CT, as well as the patient’s symptoms by the modified Rankin score (MRS) and GCS score as documented in clinical examination notes, were recorded at three-time points of interest: before the procedure, 4–6 weeks post-procedure, and 3–6 months post-procedure. Complications occurring during the admission and up to 6 months were also recorded and included bleeding requiring transfusion, hematoma at the vascular access site, worsening SDH, pseudoaneurysm/dissection of the vessel, stroke, contrast-induced kidney damage, and worsening neurological deficits despite treatment requiring additional intervention.
The primary outcome of interest was the successful treatment of the SDH, which was defined as the lesion being the same size, smaller, or if there was complete reabsorption by CT imaging by six months post procedure. The procedure was defined as a failure if the size of the SDH was larger or if the patient required surgical rescue due to an expanding hematoma. The key dependent variable was the size of the lesion/s (right, left, and midline).
Descriptive statistics, including means, standard deviations, and frequencies, were calculated for patient demographic characteristics and comorbidities, SDH characteristics, procedural details, complications, and safety and efficacy outcomes. In addition, a repeated measures analysis of variance (ANOVA) was conducted for each dependent variable (left lesion [mm], right lesion [mm], midline shift [mm], MRS, and GCS) with one within subjects’ factor time (pre, 4–6 weeks, and 3–6 months). If the repeated measures ANOVA was significant at α < 0.05, post hoc testing was conducted.
RESULTS
In our patient population, 78% (39) either had lesions improve or completely resolve.
Supplemental
No complications were experienced by any of the patients for bleeding, hematoma, worsening SDH, pseudoaneurysm, stroke, or kidney damage. However, one patient did experience a complication of worsening neurologic deficit consistent with a worsening SDH. Three patients expired before the completion of the 6-month follow-up; however, all were unrelated to the MMAE or SDH. Two of these three patients, in fact, experienced a clinical improvement following the MMAE.
Among the 50 patients, 67 lesions were present, with 24 lesions under 10 mm, 31 between 10 and 20 mm, and then 12 over 20 mm. The largest hematoma was 27 mm in width, pre-procedure, in an acute-on-cSDH. The treatment team did not utilize a cutoff value for the hematoma size but was guided by clinical presentation. Subsequently, if the patient was clinically stable despite how large the hematoma was, MMAE was still offered. Again, if there was any evidence of clinical deterioration of the patient on presentation, they were automatically excluded from MMAE as sole treatment. SDH lesion descriptive statistics over time are shown in
Based on our statistical analysis, repeated measures ANOVA demonstrated change over time with a comparison of means. The repeated measures ANOVA showed significant Cohen’s d effect sizes for the left lesion size, right lesion size, and midline shift (P < 0.05). In addition, Cohen’s d-effect sizes were small for the mean MRS and mean GCS (P > 0.05), showing no significant change over time. Specifically, for the left lesion, there was a significant decrease in size from pre to 4–6 weeks, pre to 3–6 months, and from 4–6 weeks to 3–6 months with medium to large effect sizes (d = 0.64, d = 2.01, and d = 1.08, respectively). For the right lesion, there was a significant decrease in size from pre to 3–6 months and from 4–6 weeks to 3–6 months with large effect sizes (d = 1.36 and d = 0.87, respectively) but not from pre to 4–6 weeks (P > 0.05). Finally, for the midline shift, there was a significant decrease in size from pre to 4–6 weeks, pre to 3–6 months, and from 4–6 weeks to 3–6 months with small to large effect sizes (d = 0.79, d = 1.11, and d = 0.40, respectively).
DISCUSSION
In this study of MMAE for the treatment of cSDH, acute-on-cSDH and subacute SDH, we found that the majority of the patients were successfully and safely treated. SDH is a common disease injury encountered by traumatologists and neurosurgeons. While acute SDHs generally entail observation versus evacuation based on clinical symptomatology, the presentation of acute-on-cSDH, subacute SDH, and cSDH can often be much more variable. This is most likely secondary to the overall pathophysiology of SDH as it progresses from an acute to a chronic lesion. The development of a cSDH begins as an acute SDH heals and causes dural border cells to separate, along with granulation tissue and macrophage to form.[
Various nonsurgical treatments have, therefore, been proposed to counteract this inflammatory pathophysiology. However, these treatments have not had widespread success, especially when compared to surgical evacuation.[
This study continues to show the benefits of MMAE both as a sole and adjuvant treatment option for cSDH. However, we have additionally demonstrated the safe and successful expanded application of this procedure for our patient population of acute-on-cSDH as well as a SDH with a subacute component. Our theory for the expanded application revolves around the outer membrane of a SDH that is generally derived from the dura mater. While this membrane is generally seen in cSDH, we theorize that some early formation or precursor of this membrane is also developing or developed in acute-on-cSDH and subacute SDH patients. In these SDH cases, a radiographic presence of the membrane is not always seen in CT imaging. Relying on established knowledge of physiology, we know that as a SDH ages and where persistent inflammation and bleeding exist, a SDH membrane will form. For our study, we focused on the overall aging of the SDH on imaging rather than the presence of the outer membrane. Hence, we felt that MMAE in the setting of these different SDH characteristics would also be beneficial.
While surgical evacuation remains the gold standard treatment for any aged SDH that is symptomatic, the literature and clinical practice for asymptomatic hematomas are more variable.[
Overall, our study results coincide with the literature in showing the safety and efficacy results of MMAE as a sole treatment intervention.[
Our study is limited by a retrospective design, and our treatment arm was not directly compared with a control group, such as no treatment or surgical evacuation. Furthermore, our analysis was limited by some gaps in patient follow-up that prevented further CT imaging in some cases. The results overall demonstrate the need for a larger, multicenter, randomized study to support our findings.
CONCLUSION
Our study demonstrates that MMAE is safe and successful in treating cSDH in addition to acute-on-cSDH and SDH with a subacute component. Furthermore, this can be done without increasing treatment-related complications. As a potential added benefit, our study also demonstrates the safe and efficacious use of MMAE in patients on antiplatelet or anticoagulation agents that need to be resumed somewhat acutely after intervention on the SDH. While clinical judgment and equipoise should always be of paramount importance with this disease process, it is promising for us to demonstrate the potential expanded successful application of MMAE as this procedure continues to be more widely used and accepted in the fields of neurosurgery and trauma.
Ethical approval
The research/study approved by the Institutional Review Board at Institutional Review Board at Kettering Health Network, number KHN #2021-27, dated January 14, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTAL FIGURE
Acknowledgment
We acknowledge the use of the Ohio University Heritage College Statistical Support.
References
1. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2017. 286: 992-9
2. Bounajem MT, Campbell RA, Denorme F, Grandhi R. Paradigms in chronic subdural hematoma pathophysiology: Current treatments and new directions. J Trauma Acute Care Surg. 2021. 91: e134-41
3. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Surgical management of acute subdural hematomas. Neurosurgery. 2006. 58: S16-24 discussion Si-iv
4. Chihara H, Imamura H, Ogura T, Adachi H, Imai Y, Sakai N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. 2014. 1: 1-5
5. Court J, Touchette CJ, Iorio-Morin C, Westwick HJ, Belzile F, Effendi K. Embolization of the Middle meningeal artery in chronic subdural hematoma-A systematic review. Clin Neurol Neurosurg. 2019. 186: 105464
6. Dicpinigaitis AJ, Al-Mufti F, Cooper JB, Kazim SF, Couldwell WT, Schmidt MH. Nationwide trends in middle meningeal artery embolization for treatment of chronic subdural hematoma: A population-based analysis of utilization and short-term outcomes. J Clin Neurosci. 2021. 94: 70-5
7. Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN. The surgical management of chronic subdural hematoma [published correction appears in Neurosurg Rev 2015;38:771. Anderson, Kristen (Corrected to Andersen, Kristen N)]. Neurosurg Rev. 2012. 35: 155-69
8. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
9. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg. 2019. 11: 912-5
10. Göksu E, Akyüz M, Uçar T, Kazan S. Spontaneous resolution of a large chronic subdural hematoma: A case report and review of the literature. Ulus Travma Acil Cerrahi Derg. 2009. 15: 95-8
11. Gomez-Paz S, Akamatsu Y, Salem MM, Enriquez-Marulanda A, Robinson TM, Ogilvy CS. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol. 2021. 27: 571-6
12. Haldrup M, Ketharanathan B, Debrabant B, Schwartz OS, Mikkelsen R, Fugleholm K. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir (Wien). 2020. 162: 777-84
13. Hashimoto T, Ohashi T, Watanabe D, Koyama S, Namatame H, Izawa H. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013. 4: 104
14. Ironside N, Nguyen C, Do Q, Ugiliweneza B, Chen CJ, Sieg EP. Middle meningeal artery embolization for chronic subdural hematoma: A systematic review and meta-analysis. J Neurointerv Surg. 2021. 13: 951-957
15. Jumah F, Osama M, Islim AI, Jumah A, Patra DP, Kosty J. Efficacy and safety of middle meningeal artery embolization in the management of refractory or chronic subdural hematomas: A systematic review and meta-analysis. Acta Neurochir (Wien). 2020. 162: 499-507
16. Kang J, Whang K, Hong SK, Pyen JS, Cho SM, Kim JY. Arachnoid cyst. Korean J Neurotrauma. 2015. 11: 187-90
17. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017. 101: 520-7
18. Kim HC, Ko JH, Yoo DS, Lee SK. Spontaneous resolution of chronic subdural hematoma: Close observation as a treatment strategy. J Korean Neurosurg Soc. 2016. 59: 628-36
19. Lee KS. How to treat chronic subdural hematoma? Past and now. J Korean Neurosurg Soc. 2019. 62: 144-52
20. Link TW, Boddu S, Marcus J, Rapoport BI, Lavi E, Knopman J. Middle meningeal artery embolization as treatment for chronic subdural hematoma: A case series. Oper Neurosurg (Hagerstown). 2018. 14: 556-62
21. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: A series of 60 cases. Neurosurgery. 2019. 85: 801-7
22. Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000. 93: 686-8
23. Matsumoto H, Hanayama H, Okada T, Sakurai Y, Minami H, Masuda A. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. J Clin Neurosci. 2018. 49: 40-7
24. Nakagawa I, Park HS, Kotsugi M, Wada T, Takeshima Y, Matsuda R. Enhanced hematoma membrane on Dyna CT images during middle meningeal artery embolization for persistently recurrent chronic subdural hematoma. World Neurosurg. 2019. 126: e473-9
25. Rathore L, Sahana D, Kumar S, Sahu RK, Jain AK, Tawari M. Rapid spontaneous resolution of the acute subdural hematoma: Case series and review of literature. Asian J Neurosurg. 2021. 16: 33-43
26. Roh D, Reznik M, Claassen J. Chronic subdural medical management. Neurosurg Clin N Am. 2017. 28: 211-7
27. Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A. Middle meningeal artery embolization for chronic subdural hematoma: Meta-analysis and systematic review. World Neurosurg. 2019. 122: 613-9
28. Tempaku A, Yamauchi S, Ikeda H, Tsubota N, Furukawa H, Maeda D. Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: Five cases and a review of the literature. Interv Neuroradiol. 2015. 21: 366-71
29. Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: Systematic review and case series. World Neurosurg. 2019. 126: 228-36