- Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy.
Correspondence Address:
Matteo Maria Ottaviani, Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy.
DOI:10.25259/SNI_735_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Matteo Maria Ottaviani, Maria Rossella Fasinella, Alessandro Di Rienzo, Maurizio Gladi, Lucia Giovanna Maria di Somma, Maurizio Iacoangeli, Mauro Dobran. Analysis of prognostic factors and the role of epilepsy in neurosurgical patients with brain metastases. 08-Mar-2024;15:79
How to cite this URL: Matteo Maria Ottaviani, Maria Rossella Fasinella, Alessandro Di Rienzo, Maurizio Gladi, Lucia Giovanna Maria di Somma, Maurizio Iacoangeli, Mauro Dobran. Analysis of prognostic factors and the role of epilepsy in neurosurgical patients with brain metastases. 08-Mar-2024;15:79. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12787
Abstract
Background: Brain metastases (BMs) represent the most frequent brain tumors in adults. The identification of key prognostic factors is essential for choosing the therapeutic strategy tailored to each patient. Epilepsy can precede several months of other clinical presentations of BMs. This work aimed to study the impact of epilepsy and other prognostic factors on BMs patients’ survival.
Methods: This retrospective study included 51 patients diagnosed with BMs and who underwent neurosurgery between 2010 and 2021. The impact of BM features and patient’s clinical characteristics on the overall survival (OS) was analyzed through uni- and multivariate analysis.
Results: The average OS was 25.98 months and differed according to the histology of the primary tumor. The primary tumor localization and the presence of extracranial metastases had a statistically significant impact on the OS, and patients with single BM showed a superior OS to those with multifocal lesions. The localization of BMs in the temporal lobe correlated with the highest OS. The OS was significantly higher in patients who presented seizures in their clinical onset and in those who had better post-surgical Karnofsky performance status, no post-surgical complications, and who underwent post-surgical treatment.
Conclusion: Our study has highlighted prognostically favorable patient and tumor factors. Among those, a clinical onset with epileptic seizures can help identify brain metastasis hitherto silent. This could lead to immediate diagnostic-therapeutic interventions with more aggressive therapies after appropriate multidisciplinary evaluation.
Keywords: Brain metastases, Epilepsy, Overall survival, Prognostic factors
INTRODUCTION
Brain metastases (BMs) are adults’ most common intracranial tumors, accounting for over half of brain tumors. BM incidence is rapidly increasing due to the more prolonged survival of patients with primary malignant tumors and the improvement of diagnostic tools that allow early diagnosis.[
The current BM treatments include surgery, stereotactic radiosurgery, and/or whole-brain radiation therapy. Despite remarkable advances in systemic therapies, surgery remains an essential treatment modality, especially in patients with favorable functional status, a limited number of lesions, or neurological symptoms.[
MATERIALS AND METHODS
Study population
This retrospective study included patients with BMs who underwent neurosurgical treatment at the Clinics of Neurosurgery of the University Hospital, Ospedali Riuniti of Ancona, between 2010 and 2021. Patients’ eligibility criteria were age >18 years old at the time of surgery, with a known primary cancer type, and with histologically confirmed BMs. Patients with leptomeningeal metastases and hematological malignancies were excluded from the study. The patient’s clinical characteristics analyzed were gender, age, onset symptoms, presence or absence of epileptic seizures at clinical onset, presence of extracranial secondary disorders, the time interval between BMs and primary tumor diagnosis (metachronous vs. synchronous), pre-and post-surgical Karnofsky performance status (KPS), the time elapsed between the onset of symptoms and surgical treatment, post-surgical complications, and post-surgical treatment (chemotherapy, radiotherapy, etc.). Patients were divided into three groups according to the pre-surgical KPS (100–80, 70–50, and 40–10). BMs number, localization, volume and diameters, the degree of the midline shift, and the volume of perilesional edema were analyzed as well. The measurements were carried out using the picture archiving and communication system web system on pre-surgical magnetic resonance imaging (MRI) and computed tomography scans. Tumor volumes were calculated using the spheroid volume formula: V = 4/3π × AP/2 × LL/2 × CC/2, where AP, LL, and CC were the maximum diameters in the axial (laterolateral), coronal (craniocaudal), and sagittal (anteroposterior) planes in T1-weighted MRI images after administration of gadopentetic acid (Gd-DTPA). Volume measurements were carried out in fluid-attenuated inversion recovery or T2-weighted MRI scans for the perilesional edema. Thenceforth, the edema volume (Ve) and edema index (EI) were calculated as follows: Ve = (V tumor + edema)−(V tumor); EI = (V tumor + edema)/(V tumor). The primary outcome was patients’ overall survival (OS), defined as the time interval between BM diagnosis and death from any cause.
Statistical analysis
Relationships between categorical variables were analyzed using Fisher’s exact test, whereas continuous variables were analyzed using Student’s t-test for parametric and the Mann– Whitney U-test for non-parametric data. The correlations between patients’ clinical and tumoral characteristics and the presence of seizures at onset were quantified using Spearman’s correlation coefficient (r). The OS was analyzed according to Kaplan–Meier survival curves using the log-rank test to determine statistical significance between groups and Cox regression models to assess differences in continuous variables and determine independent predictors of OS. Variables that were significantly associated with OS based on univariate analysis were subjected to multivariate analysis. Differences were regarded as significant when the probability values were <0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) ver. 20.0 (SPSS Inc., Chicago, IL).
RESULTS
Patients and tumour characteristics
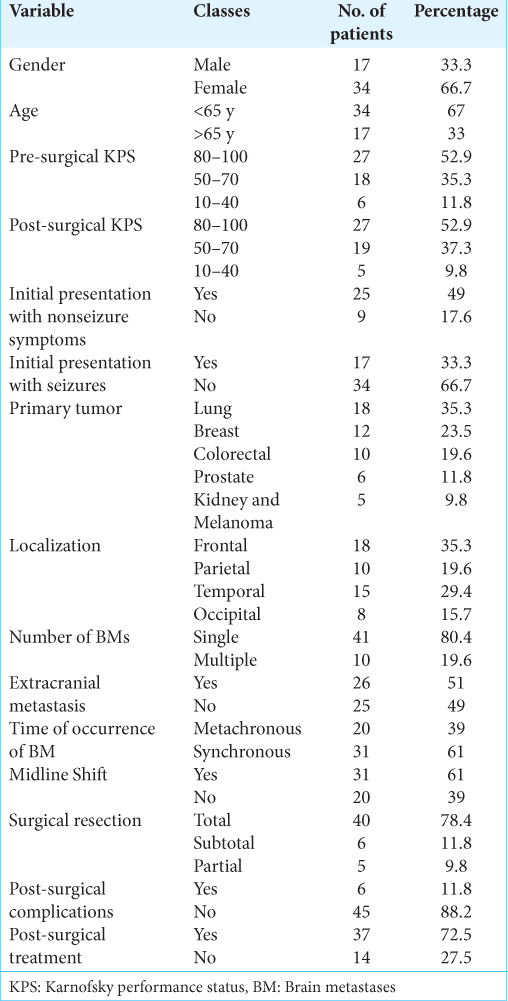
Fifty-one patients fulfilled the inclusion criteria (33.3% of males and 66.7% of females). The average age was 60.33 years, with 34/51 (67%) patients being younger than 65 years old. The follow-up available varied from a minimum of 1 month for those who died in the period immediately following surgery to a maximum of 75 months for long-term survivors. Regarding the pre-surgical KPS, 27/51 (52.9%) patients were included in the first group (100–80), 18 (35.3%) in the second (70–50), and 6 (11.8%) in the last one (40–10). A total of 25/51 (49%) patients presented nonseizure symptoms at the time of radiological diagnosis, and 17/51 (33.3%) showed seizures at the clinical onset. The primary tumors consisted of lung cancer in 18/51 (41.2%), breast cancer in 12/51 (27.5%), colorectal cancer in 10/51 (21.6%), and prostate cancer in 6/51 (11.8%) patients, whereas melanomas and renal tumors accounted for the remaining 5/51 (9.8%). Regarding their distribution, BMs were located in the frontal lobe in 18/51 (34.6%) patients, in 10/51 (30.8%) in the temporal lobe, in 8/51 (15.4%) in the occipital lobe, and 10/51 (19.2%) in the parietal lobe. Most patients showed a single BM (41/51, 80.4%), and extracranial metastases were found in 26/51 (51%) patients at the time of diagnosis. A total of 20 (39%) patients had metachronous BM from primary malignancy, whereas 31 (61%) patients had synchronous BM. The midline shift was present in 31/51 (61%) cases. The mean volume of BM was 22.68 mm2, and the average volume of perilesional edema was 96.58 mm2. All data are summarized in
Factors associated with seizures
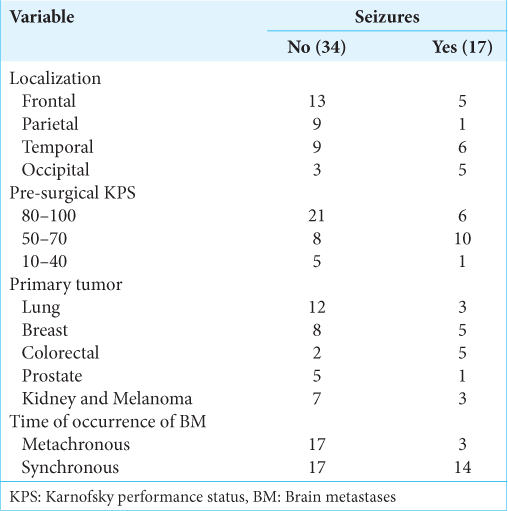
A statistically significant correlation was found between BMs localization and the presence of epilepsy at the time of diagnosis (r = 0.284, P = 0.04). The proportion of patients with seizures at the clinical onset in relationship to BMs localization is shown in
Treatment and outcome
A total of 40/51 (78.4%) patients underwent complete surgical resection, whereas the resection was partial in 5/51 (9.8%) and subtotal in 6/51 (11.8%) patients. Post-surgical complications occurred only in 6/51 (11.8%) patients and included both local (epi/subdural hematomas, hemorrhages, and wound infections) and systemic ones (pulmonary embolism, systemic infection, and hemodynamic decompensation). According to the post-surgical KPS, 27/51 (52.9%) patients were included in the first group (100–80), 19 (35.3%) in the second (70–50), and 5 (11.8%) in the last one (40–10). There was an increase of only two percentage points (from 35.3% to 37.3%) among patients with a KPS between 50 and 70 and a two-point decrease (from 11.8% to 9.8%) among patients with lower KPS than the pre-surgical KPS. Moreover, 37/51 (72.5%) patients received post-surgical treatment, whereas the remaining did not. All data are summarized in
Analysis of survival
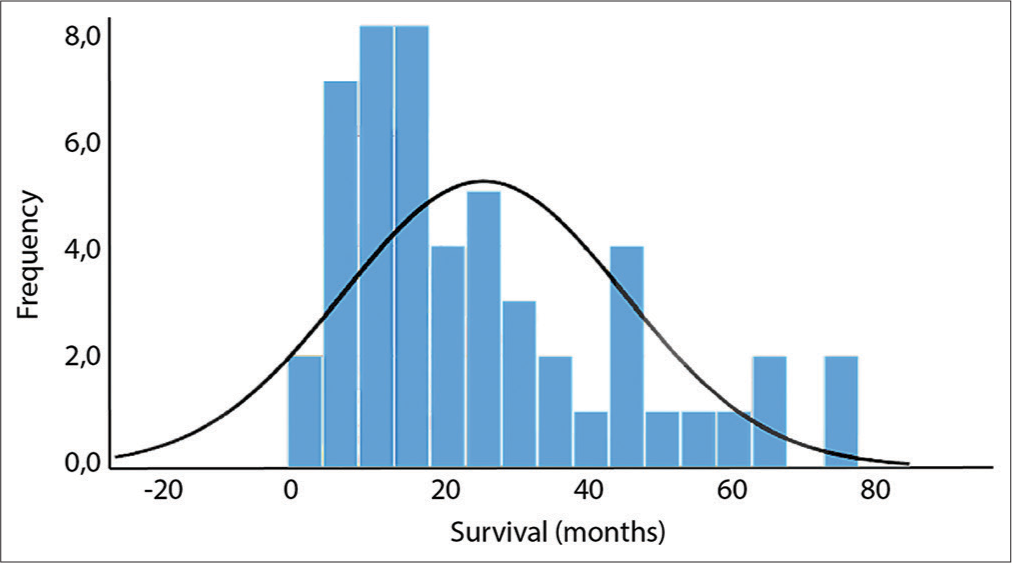
The average OS was 25.98 months with a frequency distribution, as shown in
Figure 2:
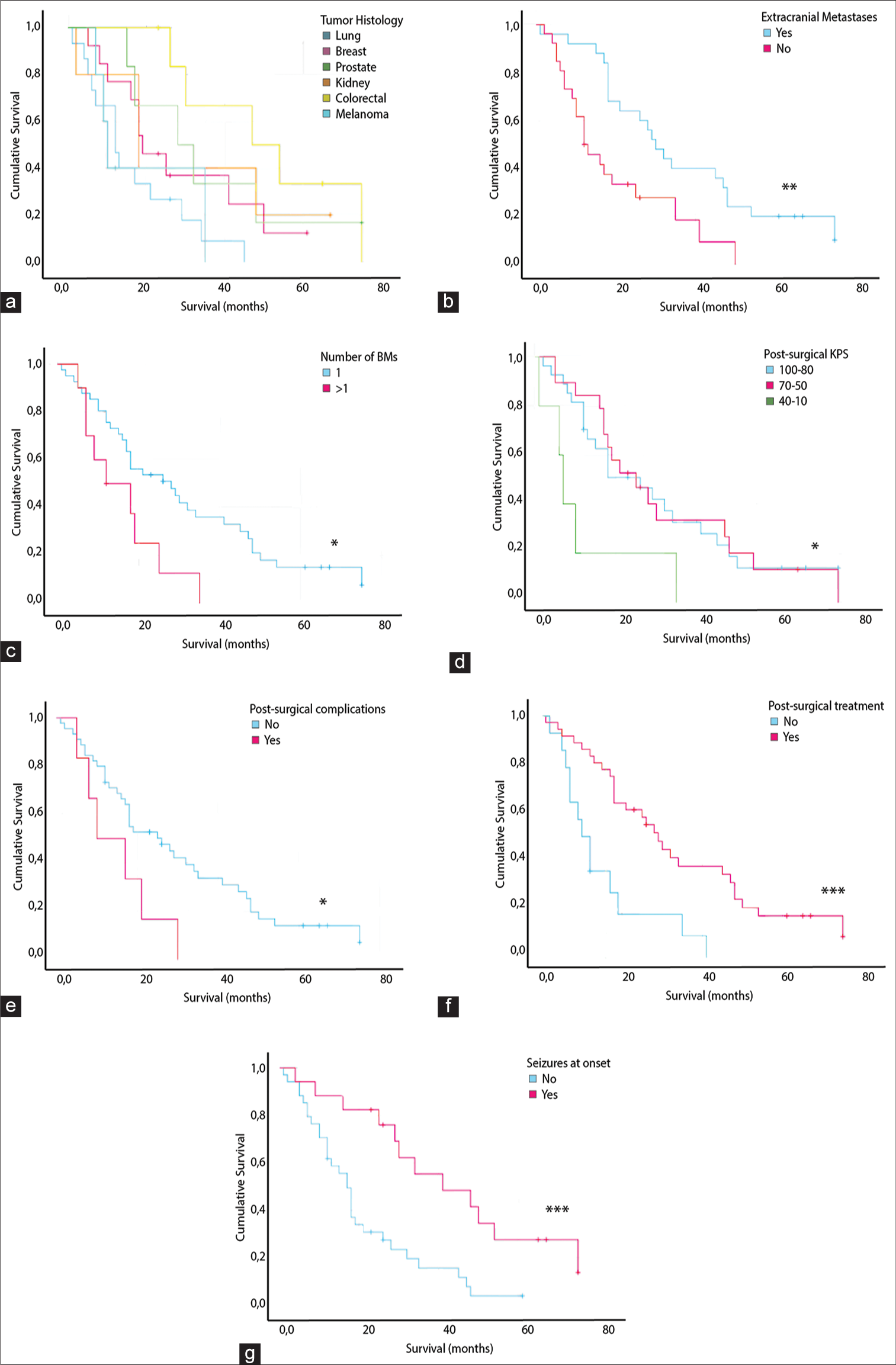
Kaplan–Meier curves with a statistically significant log-rank test of OS stratified by (a) primary histology, (b) presence of extracranial metastases (**), (c) number or BMs (*), (d) post-surgical KPS (*), (e) presence of post-surgical complications (*), (f) post-surgical treatment (***), (g) presence of seizures at the clinical onset (***).
Survival analysis with Kaplan–Meier curves showed that the presence of extracranial metastases had a statistically significant impact on the OS (P = 0.004) as it was related to an OS of 19.6 months compared to 37.2 months of patients without [
Sex, age, maximum lesion diameter, midline shift, and time elapsed between diagnosis and surgery did not show a statistically significant impact on the OS.
DISCUSSION
After the creation of the Graded Prognostic Assessment as the first objective prognostic score for patients with BMs,[
Our study highlighted the prognostically favorable role of a clinical onset with epileptic seizures. This result can be interpreted as an epileptic seizure representing a striking clinical event that requires immediate diagnostic-therapeutic intervention, and therefore, it is capable of identifying BMs hitherto unknown and silent. The percentage of patients with onset seizures was 33.3%, higher than the mean percentage of 15% found in the literature.[
CONCLUSION
The presence of BMs in the clinical history of an oncological patient usually represents a decisive moment for the patient’s prognosis and implies an accurate multidisciplinary assessment for the choice of the best therapeutic path. In this regard, identifying key prognostic factors becomes essential for choosing the best therapeutic strategy personalized to each patient. Despite being a single-center experience with a relatively small sample size, our study confirms most of the positively related prognostic factors in BM patients reported in other series. Moreover, it shows a prognostically favorable role of seizures as they promoted an earlier BM diagnosis and timelier therapeutic management that resulted in longer OS in surgically treated patients. On one side, the time of BM diagnosis should not condition too much surgical decision-making in BM patients as it did not impact patients’ OS. On the other side, it should be valued that BM resection may help in preventing seizure development or in their resolution with important improvements in patients’ quality of life and help in the better definition of the systemic disease, which may result in different or additional therapeutic implications.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chan V, Sahgal A, Egeto P, Schweizer T, Das S. Incidence of seizure in adult patients with intracranial metastatic disease. J Neurooncol 201=7;. 131: 619-24
2. Garcia JH, Morshed RA, Chung J, Millares Chavez MA, Sudhakar V, Saggi S. Factors associated with preoperative and postoperative seizures in patients undergoing resection of brain metastases. J Neurosurg. 2023. 138: 19-26
3. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 2012. 37: 745-51
4. Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000. 47: 1001-6
5. Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005. 75: 5-14
6. Gill CM, Loewenstern J, Rutland JW, Arib H, Pain M, Umphlett M. Peritumoral edema correlates with mutational burden in meningiomas. Neuroradiology. 2021. 63: 73-80
7. Gupta S, Singh S, Chophy A, Nair S, Ahuja R, Kusum K. Analysis of prognostic factors in patients with brain metastases affecting survival. J Egypt Natl Canc Inst. 2022. 34: 45
8. Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010. 96: 33-43
9. Kavouridis VK, Harary M, Hulsbergen AF, Lo YT, Reardon DA, Aizer AA. Survival and prognostic factors in surgically treated brain metastases. J Neurooncol. 2019. 143: 359-67
10. Kim YZ, Lee EH, Lee KS. Clinical analysis for brain tumor-related epilepsy during chemotherapy for systemic cancer with single brain metastasis. Cancer Res Treat. 2011. 43: 160-9
11. Kotecha R, Miller JA, Venur VA, Mohammadi AM, Chao ST, Suh JH. Melanoma brain metastasis: The impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg. 2018. 129: 50-9
12. Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003. 21: 1-23
13. Miller JA, Kotecha R, Ahluwalia MS, Mohammadi AM, Chao ST, Barnett GH. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer. 2017. 123: 2283-93
14. Miller JA, Kotecha R, Ahluwalia MS, Mohammadi AM, Suh JH, Barnett GH. The impact of tumor biology on survival and response to radiation therapy among patients with non-small cell lung cancer brain metastases. Pract Radiat Oncol. 2017. 7: e263-73
15. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-500
16. Polonara G, Aiudi D, Iacoangeli A, Raggi A, Ottaviani MM, Antonini R. Glioblastoma : A retrospective analysis of the role of the maximal surgical resection on overall survival and progression free survival. Biomedicines. 2023. 11: 739
17. Potthoff AL, Heimann M, Lehmann F, Ilic I, Paech D, Borger V. Survival after resection of brain metastasis: Impact of synchronous versus metachronous metastatic disease. J Neurooncol. 2023. 161: 539-45
18. Ricciardi S, De Marinis F. Multimodality management of non-small cell lung cancer patients with brain metastases. Curr Opin Oncol. 2010. 22: 86-93
19. Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of neuro-oncology (EANO). Neuro Oncol. 2017. 19: 162-74
20. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010. 77: 655-61
21. Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA. The prognostic value of BRAF, C-KIT, and NRAS mutations in melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017. 98: 1069-77
22. Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA. Estimating survival in melanoma patients with brain metastases: An update of the graded prognostic assessment for melanoma using molecular markers (MelanomamolGPA). Int J Radiat Oncol Biol Phys. 2017. 99: 812-6
23. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012. 82: 2111-7
24. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012. 30: 419-25
25. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A. The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys. 2016. 96: 406-13
26. Stankiewicz M, Tomasik B, Blamek S. A new prognostic score for predicting survival in patients treated with robotic stereotactic radiotherapy for brain metastases. Sci Rep. 2021. 11: 20347
27. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993. 33: 583-90
28. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: New insights and evidence-based management. Oncologist. 2014. 19: 751-9
29. Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: Use in clinical practice and trial design. Chinese Clin Oncol. 2015. 4: 1-7
30. Weller M, Stupp R, Wick W. Epilepsy meets cancer: When, why, and what to do about it?. Lancet Oncol. 2012. 13: e375-82
31. Winther RR, Vik-Mo EO, Yri OE, Aass N, Kaasa S, Skovlund E. Surgery for brain metastases-real-world prognostic factors’ association with survival. Acta Oncol (Madr). 2021. 60: 1161-8
32. Wolpert F, Lareida A, Terziev R, Grossenbacher B, Neidert MC, Roth P. Risk factors for the development of epilepsy in patients with brain metastases. Neuro Oncol. 2020. 22: 718-28
33. Wu A, Weingart JD, Gallia GL, Lim M, Brem H, Bettegowda C. Risk factors for preoperative seizures and loss of seizure control in patients undergoing surgery for metastatic brain tumors. World Neurosurg. 2017. 104: 120-8