- School of Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
- Department of Neurosurgery, The University of Oklahoma, Health Sciences Center, Oklahoma City, Oklahoma, United States.
- Department of Mathematics, The University of Texas Permian Basin, Odessa, United States.
- Department of Pediatrics, Division of Neurosurgery, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
Correspondence Address:
Ryan D. Morgan, School of Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
DOI:10.25259/SNI_471_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryan D. Morgan1, Abdurrahman F. Kharbat2, Reagan A. Collins1, John Garza3, Muhittin Belirgen4, Laszlo Nagy4. Analysis of the timing and the usage of drains following cranioplasty on outcomes and the incidence of bone resorption. 15-Sep-2023;14:329
How to cite this URL: Ryan D. Morgan1, Abdurrahman F. Kharbat2, Reagan A. Collins1, John Garza3, Muhittin Belirgen4, Laszlo Nagy4. Analysis of the timing and the usage of drains following cranioplasty on outcomes and the incidence of bone resorption. 15-Sep-2023;14:329. Available from: https://surgicalneurologyint.com/surgicalint-articles/12558/
Abstract
Background: Pediatric cranioplasty is associated with a high rate of complications, including bone resorption (BR) in 20–50% of cases. We aimed to evaluate factors contributing to BR, including the effect of the timing of cranioplasty and the use of post-surgical drains.
Methods: This is a dual institution retrospective review of all patients under 18 years old who underwent a cranioplasty following a decompressive craniectomy (DC) for the treatment of traumatic brain injury between 2011 and 2021. Early cranioplasty was defined as within 30 days after DC and late cranioplasty as >30 days. Patients were grouped by BR and separately by timing to cranioplasty. Groups were compared based on the Glasgow Outcome Scale (GOS) and postoperative drain usage.
Results: A total of 30 patients were included in the study. The mean age was 7.39 (standard deviation = 6.52) and 60% were male. The median time to cranioplasty was 13 days (interquartile range = 10–17). BR was present in 16.7% of cases. A subgaleal drain was utilized in 93.3% and an external ventricular drain (EVD) in 63.3% of patients following cranioplasty. Drain usage was not associated with BR and timing to cranioplasty was not associated with discharge or 6-month GOS.
Conclusion: This study demonstrates that early cranioplasty following DC may have similar outcomes to late cranioplasty. Post-surgical EVDs and subgaleal drains did not increase the incidence of BR, suggesting their importance in the postoperative management of these patients.
Keywords: Bone resorption, Cranioplasty, Decompressive craniectomy, External ventricular drains, Hydrocephalus
INTRODUCTION
Decompressive craniectomy (DC) is a potentially lifesaving procedure that is used to relieve elevated intracranial pressure (ICP) or to evacuate lesions producing symptoms from mass effect following injuries such as severe traumatic brain injury (TBI), stroke, or encephalitis.[
In studies looking at the incidence and risk factors for postoperative complications of cranioplasty, there are conflicting results with a paucity of data determining risk factors and methods of prevention.[
MATERIALS AND METHODS
Study population
This is a retrospective review of patient data at two medical institutions between the years 2011 and 2021. Patients met inclusion criteria if they were younger than 18 years of age, underwent a DC due to TBI, and underwent a subsequent cranioplasty. Both procedures (DC and cranioplasty) had to be completed at these institutions, as procedures at outside hospitals were excluded. Patients who received a DC for an indication other than TBI were also excluded from the study.
Data acquisition
All data were recorded from the existing electronic chart records including nursing notes, physician notes, operative notes, and imaging studies. The initial type of injury was categorized as closed head injury, skull fracture, and penetrating head injury. Mechanism of injury was categorized as a motor vehicle collision, known accidental, non-accidental trauma (NAT), unknown, and a gunshot wound. Indications for DC were defined as herniation, intractable ICP, midline shift, declining Glasgow Coma Scale/neurologic deterioration, subarachnoid space decompression, subarachnoid space compression, vascular compression, stroke, anoxic brain injury, and hemorrhage. Type of DC was grouped as right/ left frontal, right/left parietal, left/right temporal, right/left frontotemporoparietal, bifrontal, bilateral frontotemporal, bilateral parietal, and occipital. Data were subsequently collected for the indications for and type of DC. Other DC data collected were the material utilized for the duraplasty as well as the size of the skull defect following DC. The skull defect size was measured utilizing the AC method that has previously been described.[
Patients were retrospectively divided into early and late cranioplasty groups based on operation timing post-DC. Patients who had a cranioplasty in fewer than 30 days after DC were in the early group and patients who had their cranioplasty more than 30 days after DC were in the late group. The decision on timing of cranioplasty was based on imaging results, ICP, softness of craniectomy site, patient neurological and overall progression, and ultimately at the discretion of the operating neurosurgeon. DC surgical site infection was a contraindication to early cranioplasty. Patients were also retrospectively divided into groups based on the presence of BR. The two patient groups were compared using all other variables. BR was determined based on imaging studies [
Figure 1:
Computed tomography scan depicting bone resorption 1-year following native cranioplasty with a right-sided frontotemporoparietal cranioplasty. (a and b) Axial views of bone resorption. (c) Coronal view demonstrating bone resorption on the right side. (d) Sagittal view demonstrating bone resorption.
Complications were recorded when there was a need for a follow-up operation. Complications were measured from the operation to the patient’s last follow-up appointment. Any incidence of epidural or subdural hematomas, subdural fluid collection, wound healing disturbance, subgaleal fluid collection, abscess formation, surgical site infection, CSF leak, hydrocephalus requiring a ventriculoperitoneal shunt (VPS), and BR were reported.
All patients had a follow-up period of 6 months. Due to the rural population served by these institutions, longer follow-up periods were not consistently met so to keep the follow-up period equal between all patients, a maximal interval of 6 months was used when measuring functional outcome. Outcomes were measured at discharge, 3-month follow-up, and 6-month follow-up using the previously described Glasgow Outcome Score (GOS). Positive outcomes were defined as s GOS of 4 and 5 indicating moderate, or mild to no disability respectively with preserved patient independence. GOS of 3 was defined as severe disability with loss of independence, 2 as a persistent vegetative state, and GOS of 1 indicated death. Patients were followed for their entire follow-up period for complications.
Statistical analysis
The project is a retrospective and exploratory analysis using descriptive and inferential statistics with an emphasis on hypothesis tests. The project compares the characteristics and outcomes of patients partitioned into two groups. Statistical analyses used two-sided p-values, independent samples, and a significance level of α = 0.05. Adjustments to P-values to control the family-wise error rate and the false-positive rate have not been made since the study intends to evaluate the plausibility of significant differences and involves large numbers of supporting covariates but only two exposure variables and two primary outcome variables. Confidence intervals for effect sizes and population parameters are not reported since hypothesis testing is the inferential area of focus and limited sample size implies large confidence intervals. Interval level variables are summarized using the mean and standard deviation (SD) while categorical variables are summarized using counts and percentages. Differences in interval level variables are tested using the permutational unequal variance Welch t-test based on 1000 permutations. Differences in nominal level and binary variables are tested using Fisher’s test. The standardized mean difference is used as the standardized effect size for nominal-level, binary, and interval-level variables. Differences in ordinal level variables are tested using the Mann–Whitney U-test with Cliff ’s δ as the standardized effect size. To aid in the interpretation of nonsignificant P-values, supplementary
RESULTS
Demographics
A total of 47 patients who underwent a DC were identified; 30 patients met the final inclusion criteria. At 6-month follow-up, two patients were lost. Of the 30 patients, 18 (60.0%) were male and the mean patient age was 7.39 (SD = 6.53) years. The most common etiology of these patients was motor vehicle collision (n = 10, 33.3%), followed by known accidental (n = 6, 20.0%), NAT (n = 8, 26.7%), and gunshot wound (n = 6, 20.0%). There were no mortalities in this patient group. The median time to cranioplasty was 13 days with 80.0% (n = 24) of patients undergoing cranioplasty in under 30 days.
Outcomes
Mean GOS at discharge was 3.73 (SD = 0.91) with 26.7% (n = 8) of patients having minor to no disability, 23.3% (n = 7) having a moderate disability, 46.7% (n = 14) of patients with severe disability, and 3.3% (n = 1) patients in a persistent vegetative state. At 6-month follow-up, the mean GOS was 4.47 (SD = 0.90) with 57.1% (n = 16) of patients having mild to no disability, 21.4% (n = 6) of patients having moderate disability, 17.9% (n = 5) of patients having severe disability, and 3.6% (n = 1) patients in a persistent vegetative state. At 6-month follow-up, 78.6% (n = 22) had favorable outcomes, GOS 4 or 5. The overall complication rate following cranioplasty was 36.0% (n = 11). The most common complications encountered were surgical site infection (16.7%), BR (16.7%), and wound healing disturbance (13.3%). Hydrocephalus occurred in one patient (3.3%) and one patient (3.3%) had a subdural and subgaleal fluid collection. Patients were followed for a mean of 25.2 (SD = 23.81) months following cranioplasty for complications. Patient age, indication for original DC, and timing of DC were not associated with outcome.
Bone resorption
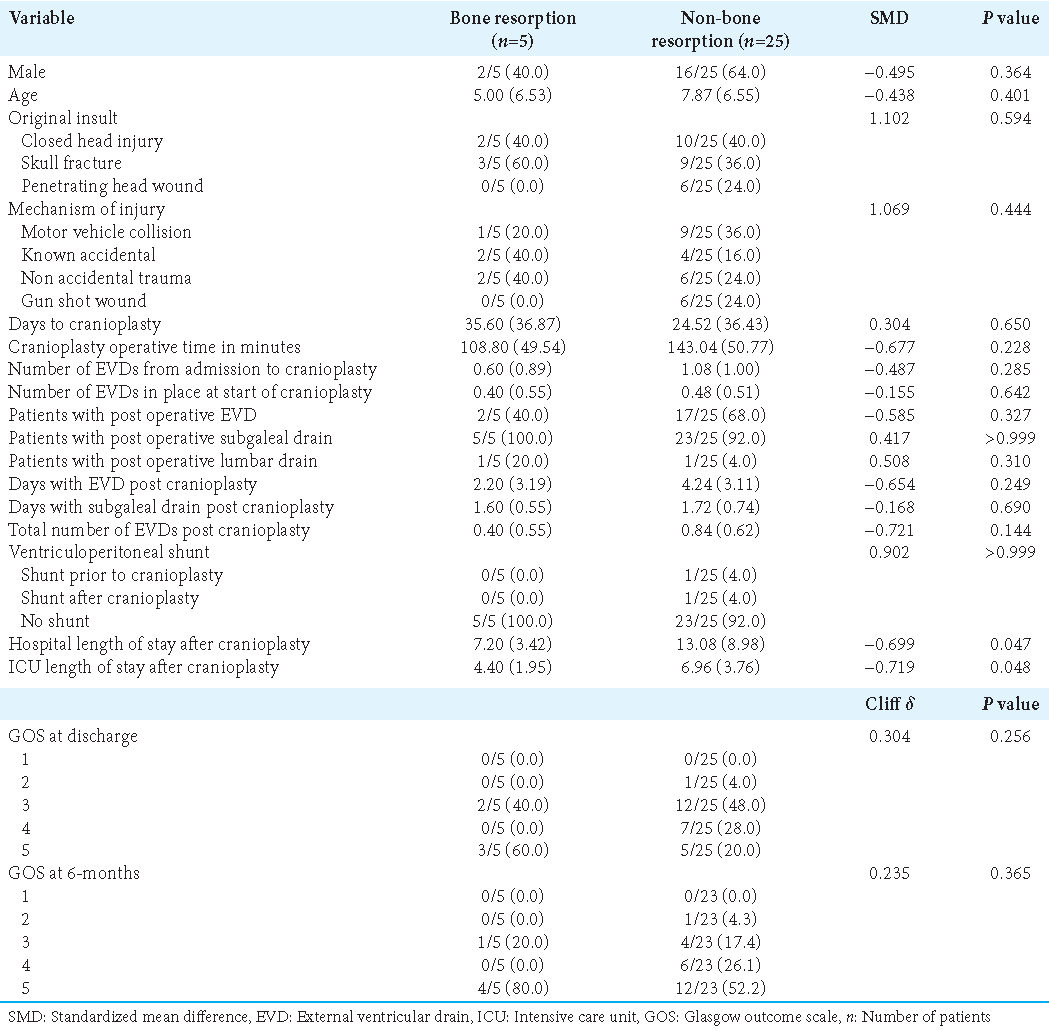
BR occurred in a total of 5 (16.7%) patients. The mean time from discharge to BR was 12 months (SD: 7.3). Age, gender, injury, type of DC, and indication for DC were not associated with BR [
Timing of cranioplasty
Of the 30 patients, 24 (80.0%) underwent an early cranioplasty (<30 days) and 6 (20.0%) underwent a late cranioplasty [
Postsurgical drains
A postoperative drain was used in all patients in the study. A subgaleal drain was utilized in 93.3% (n = 28) of patients following their operation. EVDs were also present in 63.3% (n = 19) of patients. A lumbar drain was used in two patients (6.7%) and a VPS was inserted in two patients (6.7%), one before cranioplasty and one at 3-month follow-up. The use of EVDs and subgaleal drains was not associated with BR or postoperative surgical site infection (P = 0.327 and P > 0.999, respectively). No patients in the late cranioplasty group received an EVD after cranioplasty; EVDs were only present in patients undergoing early cranioplasty.
DISCUSSION
After undergoing, DC patients must undergo a cranioplasty in which the skull flap is replaced, thus protecting the brain, restoring cosmesis, and restoring homeostatic CSF hydrodynamics, all of which aid in overall brain recovery.[
During cranioplasty, the replaced bone flap may be either autologous, synthetic, or bioprosthetic. Autologous bone flaps are typically the preferred choice, especially in pediatric patients as their skull is still growing and developing.[
BR is the most encountered complication following cranioplasty, with rates ranging from 20 to 50% in prior literature.[
Postoperative drains, particularly EVDs, lack data surrounding their indication and usage. A previous study conducted by Rocque et al. reported that EVD use after cranioplasty was a significant risk factor for BR. However, as the study noted, this may be misleading as the sample size was low and the confidence intervals were wide.[
We speculate that in addition to subgaleal drains, EVDs work to decrease the incidence of subgaleal fluid collections. The absence of fluid in this space decreases overall pressure on the bone flap; thus, the bone is more readily vascularized and integrated into the skull. This could allow for a more robust healing process and decreased resorption. We believe that this, in part, may have contributed to our lower rate of resorption. With all but two patients in this study receiving a subgaleal drain, it is difficult to determine the effect the drain usage which had on BR. Furthermore, the incidence of drain use is not well reported in the literature making it difficult to determine the possible influence the drains had on BR.
The importance of timing to cranioplasty on BR and overall outcome within the pediatric population has yet to be elucidated. Several studies have concluded that timing to cranioplasty after DC does not affect BR or other complications in pediatric patients.[
DC and cranioplasty are known to alter CSF hydrodynamics within the brain, resulting in higher rates of hydrocephalus.[
Limitations
A limitation of this study is the retrospective nature resulting in a lack of randomization which can include bias. Due to the retrospective nature, there was not a consistent set time point for every patient who received follow-up imaging. Due to the numerous covariates and limited sample size, we were unable to utilize multivariable regression analysis to determine the independent predictors of BR. We have dichotomized time to cranioplasty, a continuous variable, into a binary variable. For larger studies, it may be preferable to measure the association of time with cranioplasty and BR using methods such as logistic regression. P-values have not been adjusted for multiplicity and should not be used to infer definitive effects. In addition, the methodologic constraints and small sample size (n = 30) limit the study’s generalizability. Type II errors cannot be completely ruled out due to the pilot scale of the samples included in the study. However, this study has a comparable number of patients with the previous published literature and provides a comprehensive assessment of pediatric patients undergoing early and late DC at our institution.[
CONCLUSION
In this present study, the timing of cranioplasty did not influence outcome or BR indicating that early cranioplasty is a suitable option when patients are clinically stable. BR was not increased by our use of drains or early cranioplasty. This study was one of the larger studies looking at cranioplasties in pediatric patients and has the lowest reported incidence of BR.
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abu-Ghname A, Banuelos J, Oliver JD, Vyas K, Daniels D, Sharaf B. Outcomes and complications of pediatric cranioplasty: A systematic review. Plast Reconstr Surg. 2019. 144: 433.e
2. Akins PT, Guppy KH. Are hygromas and hydrocephalus after decompressive craniectomy caused by impaired brain pulsatility, cerebrospinal fluid hydrodynamics, and glymphatic drainage? Literature overview and illustrative cases. World Neurosurg. 2019. 130: e941-52
3. Beez T, Munoz-Bendix C, Ahmadi SA, Steiger HJ, Beseoglu K. From decompressive craniectomy to cranioplasty and beyond-a pediatric neurosurgery perspective. Childs Nerv Syst. 2019. 35: 1517-24
4. Bowers CA, Riva-Cambrin J, Hertzler DA, Walker ML. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013. 11: 526-32
5. Bykowski MR, Goldstein JA, Losee JE. Pediatric cranioplasty. Clin Plast Surg. 2019. 46: 173-83
6. Carballo-Cuello C, de Jesus O, Fernandez-de Thomas RJ, Garcia M, Vigo-Prieto J, de Jesus-Espinosa A. Posttraumatic hydrocephalus in pediatric patients after decompressive craniectomy. World Neurosurg. 2020. 136: e690-4
7. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: Magnetic resonance imaging quantitative analysis. Neurol Res. 1997. 19: 311-6
8. Frassanito P, Massimi L, Caldarelli M, Tamburrini G, Di Rocco C. Complications of delayed cranial repair after decompressive craniectomy in children less than 1 year old. Acta Neurochir (Wien). 2012. 154: 927-33
9. Fu KJ, Barr RM, Kerr ML, Shah MN, Fletcher SA, Sandberg DI. An outcomes comparison between autologous and alloplastic cranioplasty in the pediatric population. J Craniofac Surg. 2016. 27: 593-7
10. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004. 100: 163-8
11. Greitz D. Radiological assessment of hydrocephalus: New theories and implications for therapy. Neurosurg Rev. 2004. 27: 145-65
12. Hersh DS, Anderson HJ, Woodworth GF, Martin JE, Khan YM. Bone flap resorption in pediatric patients following autologous cranioplasty. Oper Neurosurg (Hagerstown). 2021. 20: 436-43
13. Ho MY, Tseng WL, Xiao F. Estimation of the craniectomy surface area by using postoperative images. Int J Biomed Imaging. 2018. 2018: 5237693
14. Jo K, Joo WI, Yoo DS, Park HK. Clinical significance of decompressive craniectomy surface area and side. J Korean Neurosurg Soc. 2021. 64: 261-70
15. Josan VA, Sgouros S, Walsh AR, Dover MS, Nishikawa H, Hockley AD. Cranioplasty in children. Childs Nerv Syst. 2004. 21: 200-4
16. Khanna O, Baldassari MP, Al Saiegh F, Mouchtouris N, Ghosh R, Theofanis TN. Ultrasound-guided ventricular puncture during cranioplasty. World Neurosurg. 2021. 146: e779-85
17. Klieverik VM, Miller KJ, Singhal A, Han KS, Woerdeman PA. Cranioplasty after craniectomy in pediatric patients-a systematic review. Childs Nerv Syst. 2019. 35: 1481-90
18. Malcolm JG, Mahmooth Z, Rindler RS, Allen JW, Grossberg JA, Pradilla G. Autologous cranioplasty is associated with increased reoperation rate: A systematic review and meta-analysis. World Neurosurg. 2018. 116: 60-8
19. Manfiotto M, Beccaria K, Rolland A, Paternoster G, Plas B, Boetto S. Decompressive craniectomy in children with severe traumatic brain injury: A multicenter retrospective study and literature review. World Neurosurg. 2019. 129: e56-62
20. Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014. 156: 813-24
21. Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 2012. 10: 268-72
22. Rocque BG, Agee BS, Thompson EM, Piedra M, Baird LC, Selden NR. Complications following pediatric cranioplasty after decompressive craniectomy: A multicenter retrospective study. J Neurosurg Pediatr. 2018. 22: 225-32
23. Rocque BG, Amancherla K, Lew SM, Lam S. Outcomes of cranioplasty following decompressive craniectomy in the pediatric population. J Neurosurg Pediatr. 2013. 12: 120-5
24. Salam AA, Ibbett I, Thani N. Paediatric cranioplasty: A review. Interdiscip Neurosurg. 2018. 13: 59-65
25. Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V. Cranioplasty after decompressive craniectomy: The effect of timing on postoperative complications. J Neurotrauma. 2012. 29: 1090-5
26. Sobani ZA, Shamim MS, Zafar SN, Qadeer M, Bilal N, Murtaza SG. Cranioplasty after decompressive craniectomy: An institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011. 2: 123
27. Zaccaria L, Tharakan SJ, Altermatt S. Hydroxyapatite ceramic implants for cranioplasty in children: A single-center experience. Childs Nerv Syst. 2017. 33: 343-8