- Department of Neurological Surgery, Fundación Universitaria de Ciencias de la Salud (FUCS), Hospital Infantil Universitario de San José, Bogotá, Colombia,
- LINT, Facultad de Medicina, Universidad Nacional de Tucumán, Argentina,
- Department of Neurological Surgery, Hospital Padilla, Tucumán, Argentina,
- Department of Clinical-Surgical, Diagnostic and Pediatric Sciences, Neurosurgery Unit, University of Pavia, Pavia, Italy,
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, School of Medicine, University of Buenos Aires, Buenos Aires, Argentina,
- Department of Neurological Surgery, Hospital San Fernando, Buenos Aires, Argentina,
- Department of Surgical Sciences, Neurosurgery Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Correspondence Address:
Sabino Luzzi, Department of Neurosurgery, University of Pavia, Pavia, Italy.
DOI:10.25259/SNI_404_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nadin J. Abdala-Vargas1, Hernando A. Cifuentes-Lobelo1, Edgar Ordoñez-Rubiano1, Javier G. Patiño-Gomez1, Juan F. Villalonga2,3, Alice Giotta Lucifero4, Alvaro Campero2,3, Valeria Forlizzi5, Matías Baldoncini5,6, Sabino Luzzi4,7. Anatomic variations of the floor of the third ventricle: Surgical implications for endoscopic third ventriculostomy. 27-May-2022;13:218
How to cite this URL: Nadin J. Abdala-Vargas1, Hernando A. Cifuentes-Lobelo1, Edgar Ordoñez-Rubiano1, Javier G. Patiño-Gomez1, Juan F. Villalonga2,3, Alice Giotta Lucifero4, Alvaro Campero2,3, Valeria Forlizzi5, Matías Baldoncini5,6, Sabino Luzzi4,7. Anatomic variations of the floor of the third ventricle: Surgical implications for endoscopic third ventriculostomy. 27-May-2022;13:218. Available from: https://surgicalneurologyint.com/surgicalint-articles/11625/
Abstract
Background: Endoscopic third ventriculostomy (ETV) is currently used as a treatment for different types of hydrocephalus. However, the anatomical endoscopic variants of the third ventricle floor (3VF), as well as their surgical implications, have been underrated. The anatomic variations of the 3VF can influence the technique and the success rate of the ETV. The purpose of this article is to describe the anatomical variations of 3VF, assess their incidence, and discuss the implications for ETV.
Methods: Intraoperative videos of 216 patients who underwent ETV between January 2012 and February 2020 at Hospital Infantil Universitario de San José, Bogotá, Colombia were reviewed. One hundred and eighty patients who met the criteria to demonstrate the type of 3VF were selected.
Results: 3VF types were classified as follows: (1) Thinned, (2) thickened, (3) partially erased, (4) globular or herniated, and (5) narrowed.
Conclusion: Knowledge of anatomical variations of the 3VF is paramount for ETV and it influences the success rate of the procedure.
Keywords: Cerebrospinal fluid shunt, Endoscopic third ventriculostomy, Hydrocephalus, Neuroendoscopy, Ventriculostomy
INTRODUCTION
The third ventricle floor (3VF) is a complex anatomical region made up of multiple structures of great importance for endocrinological and executive functions. From rostral to caudal, it involves the supraoptic recess, optic chiasm, infundibulum, tuber cinereum, and mammillary bodies [
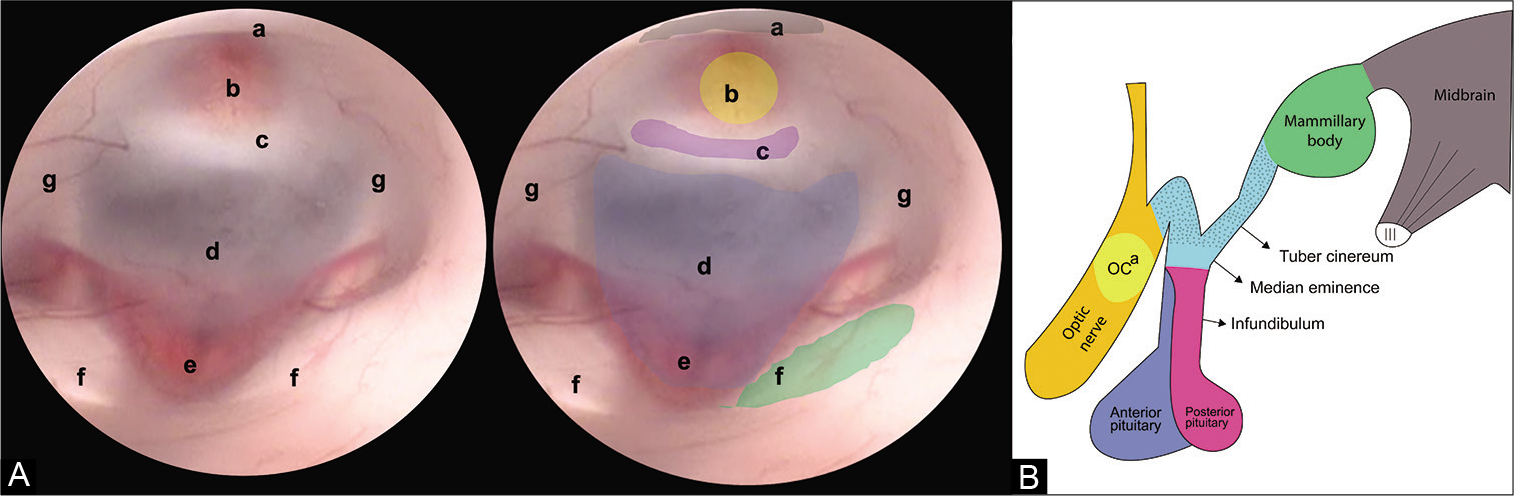
Figure 1:
(A) Endoscopic view of the FTV. In this image, the anatomical structures that make up the FTV are recognized, from anterior to posterior we find. (a) Optic chiasm, (b) infundibulum, (c) dorsum sellae, (d) floor of the third ventricle (tuber cinereum), (e) basilar complex, (f) mammillary bodies, and (g) hypothalamic wall. (B) Lateral view of the diencephalon. The structures that make up the floor of the third ventricle can be appreciated. (OCa) Optic chiasm.
First described by Mixter et al. in 1923, the endoscopic third ventriculostomy (ETV) is the technique routinely used for the treatment of obstructive hydrocephalus.[
Despite the identification of these anatomical structures, the anatomy of the 3VF is variable. These variations were briefly studied, especially in relation to complications rate and failures of the surgical procedure. In 2016, Sughrue et al. published the first study about the most common variations of the endoscopic anatomy, during ETV, on a series of 50 operated patients.[
On these assumptions, the present image report aims to contribute to the knowledge of endoscopic anatomical variations of the 3VF and its probable incidence and proposes two de novo variants, founded on an extensive series of illustrated cases. The surgical implications of these variations in ETV were further discussed.
MATERIALS AND METHODS
Intraoperative videos of 216 patients undergone ETV between January 2012 and February 2020 at the Hospital Infantil Universitario de San José, Bogotá, Colombia were reviewed. One hundred and eighty patients who met the criteria were chosen to demonstrate the type of 3VF taking into account the quality of the recording. All procedures were performed with Karl Storz ventricular endoscopes (Tuttlingen, Germany).
The videos were reviewed together with the clinical history of each patient, as well as the reports of the surgical procedure, considering their age, sex, history, and whether they required reoperation for the management of hydrocephalus. From each neuroendoscopy video, the 3VF image was captured. From each image obtained, the anatomical structures were identified, labeled, and edited using CorelDRAW Graphics Suite 2020 (Corel TM, Ottawa, Canada).
Beyond a detailed description of the 3VF anatomy [
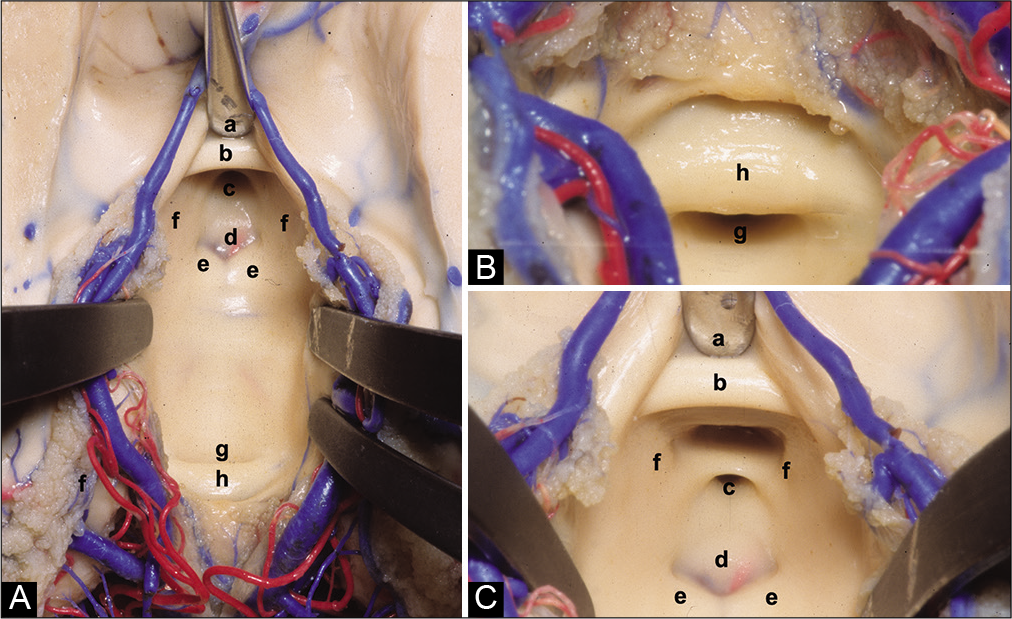
Figure 2:
(A) Anatomical superior view of the III ventricle floor. (B) Posterior superior view of the III ventricle floor. (C) Anterior superior view of the III ventricle floor. (a) Chiasmatic recess, (b) anterior commissure, (c) infundibular recess, (d) tuber cinereum, (e) mammillary bodies, (f) medial wall of the hypothalamus, (g) aqueduct, and (h) posterior commissure.
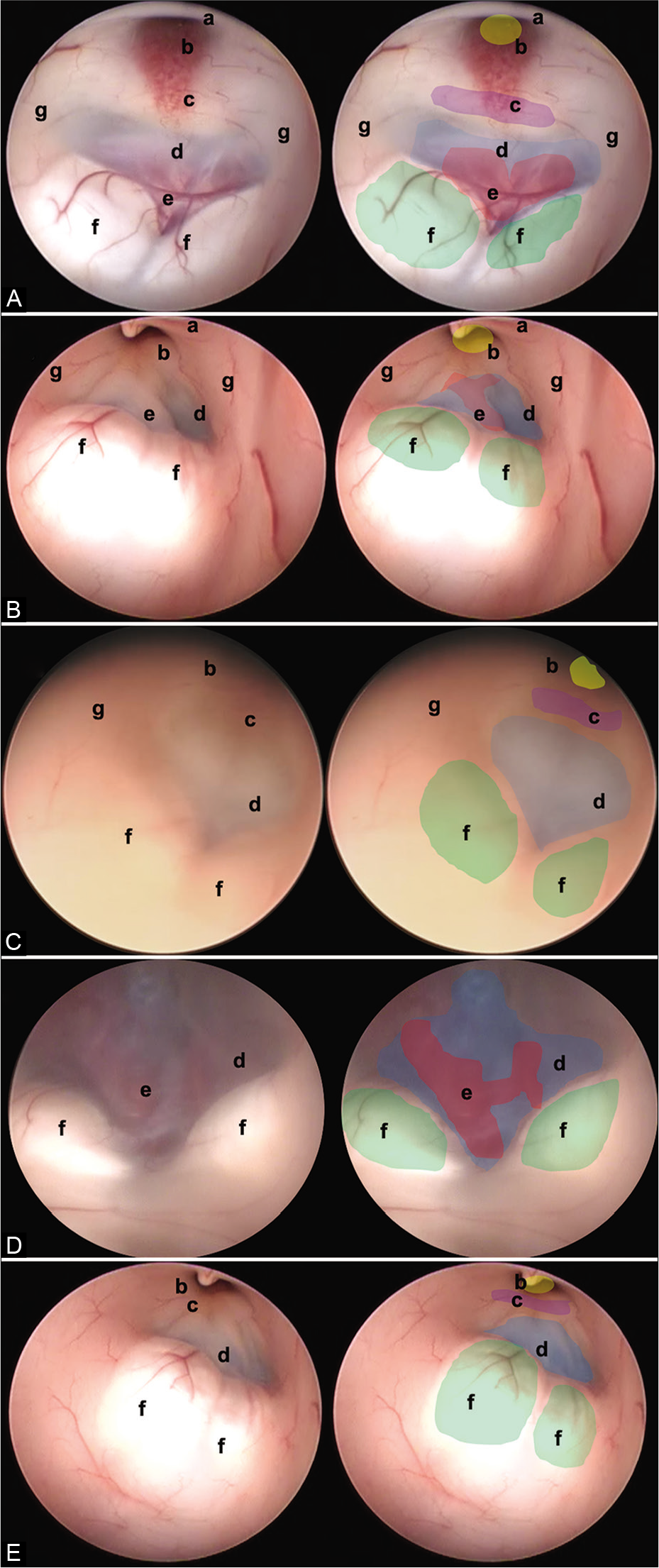
Figure 3:
In this figure, it is possible to identify the different endoscopic anatomical variants of the FTV, in each image, a complete view of the ventricle is shown, and next to it is the representation of each of its structures. (A) Thinned floor, (B) thickened floor, (C) cleared floor, (D) globus or herniated floor, and (E) narrow floor. The anatomical structures are found as follows: (a) optic chiasm (black), (b) infundibulum (yellow), (c) dorsum sellae (purple), (d) floor of the third ventricle, (tuber cinereum) (blue), (e) basilar complex (red), (f) mammillary bodies (green), and (g) hypothalamic wall.
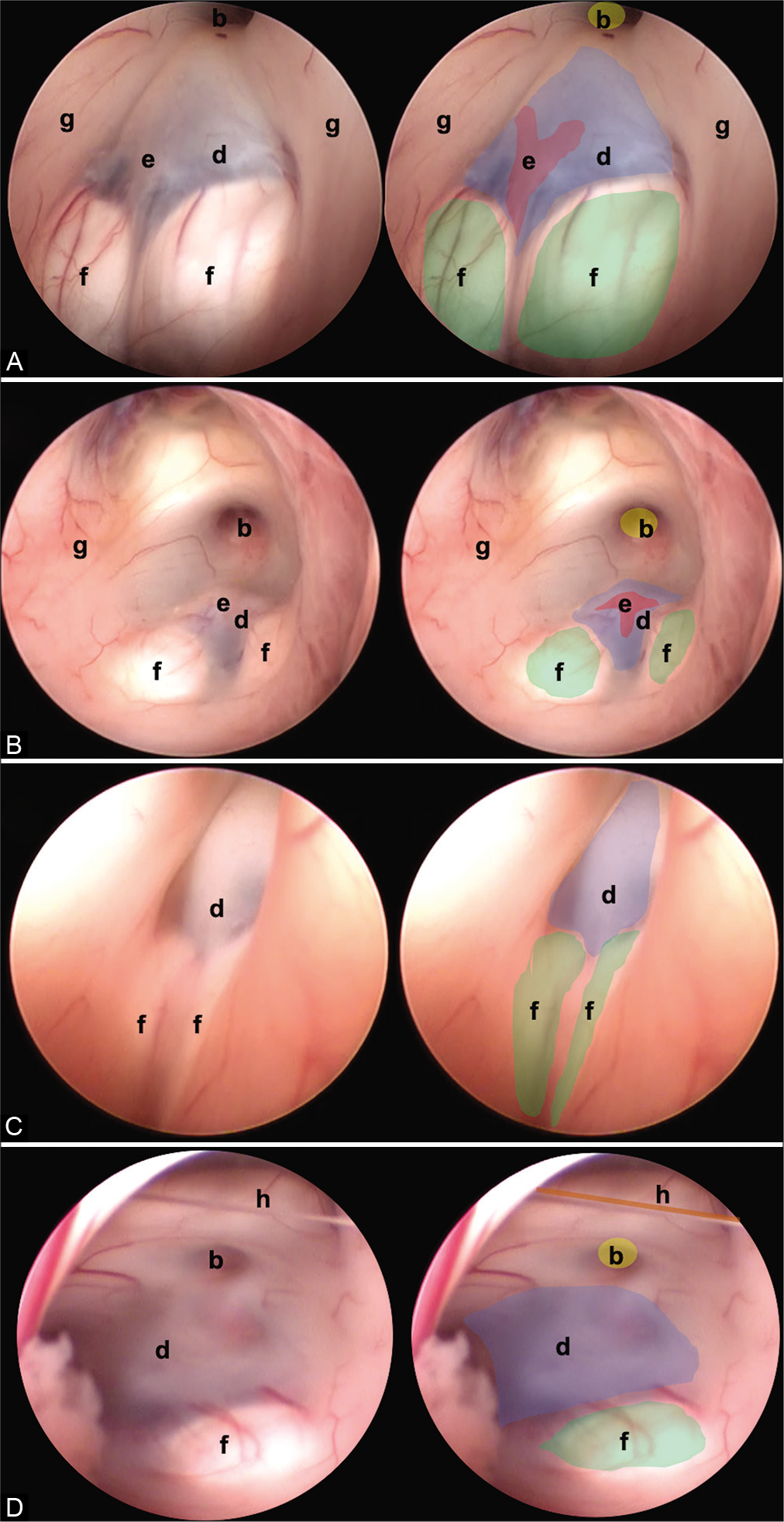
Figure 4:
Other anatomic variants independent of the type of TVF can be identified. (A) Separate mammillary bodies, (B) the prominent basilar artery, (C) elongated third ventricle, and (D) interthalamic bands. The anatomical structures are found as follows: (b) infundibulum (yellow), (d) floor of the third ventricle (tuber cinereum) (blue), (e) basilar complex (red), (f) mammillary bodies (green), and (g) hypothalamic wall.
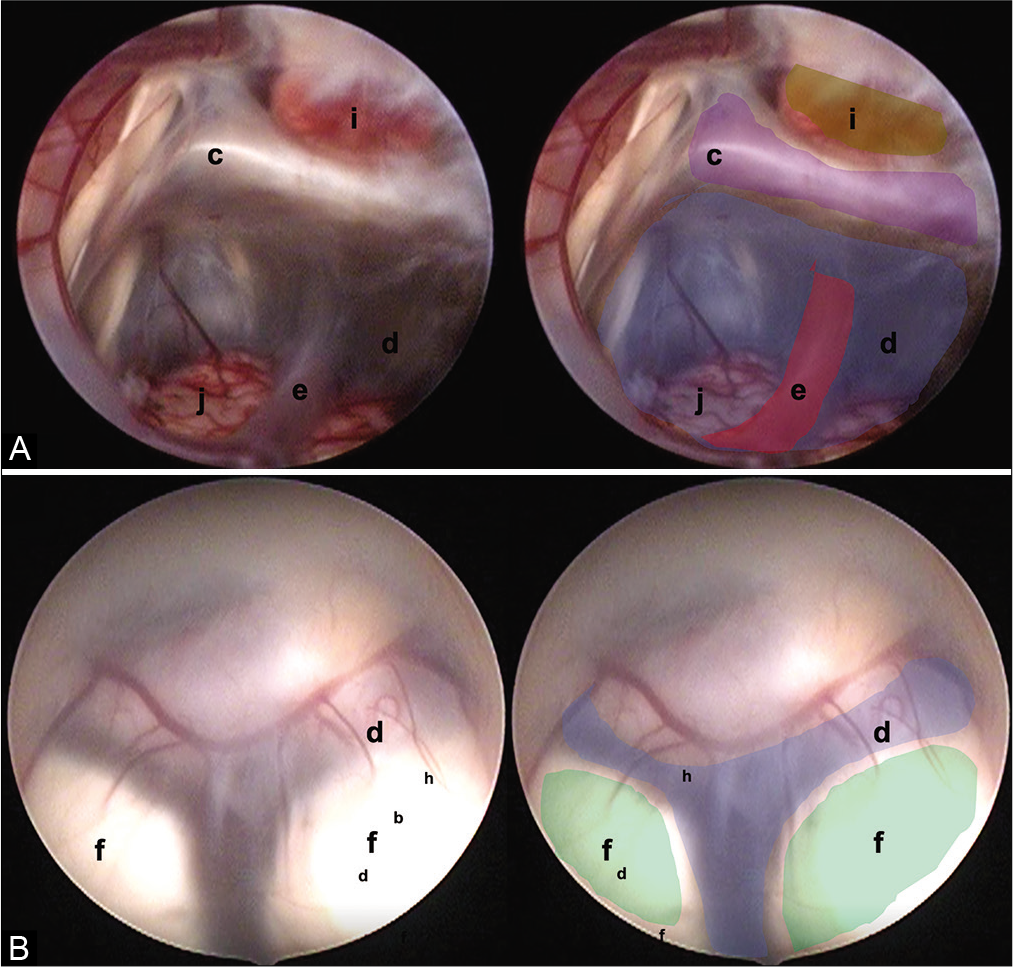
Figure 5:
Two endoscopic anatomical variants are presented that we consider are not reported in the medical literature and that can constitute a surgical challenge for the surgeon in charge. (A) Translucent floor, (B) Y-floor. Anatomical structures are found as follows: (c) Dorsum sellae (purple), (d) floor of the third ventricle (tuber cinereum) (blue), (e) basilar complex (red), (f) mammillary bodies (green), (i) pituitary, and (j) brainstem.
The statistical analysis was carried out using the Epi-Info 7.2.3.1 software.
RESULTS
Of the 180 operated patients, 57% were women, and 49% were in the context of acute hydrocephalus. It was observed that 22% of the patients had thinned floors and its association with chronic hydrocephalus was estimated at 37%. The thickened floor represented 53% of our series and a relationship of 47% was estimated with patients previously operated on. The globose floor was only present in 6% of the patients, the partially erased floor in 7% of the patients, and the configuration narrow floor was found in 12% of patients. The reoperation rate was 13%. The number of patients with ventricular bypass systems before 3VF was 56.49%.
DISCUSSION
Understanding the neurosurgical anatomy and its variations are of great importance for neurosurgical practice.[
Anatomic variations of 3VF
Thinned floor
Thinned 3VF is characterized by a thin and transparent membrane and may occur in patients with chronic hydrocephalus.[
Thickened floor
A 3VF with a thick membrane associated with inflammatory pathologies or long-standing hydrocephalus processes was found.[
Partially erased floor
There is only one description of this type of anatomical variation in the literature.[
Globose or herniated floor
This kind of variant of 3VF was the most infrequent in our series and had little relation to the presence of chronic hydrocephalus (7%) although it is described as a cause due to pressure changes in the third ventricle. Some authors show ballooning of the 3VF after perforation of the tuber cinereum[
Narrow floor
The intraoperative image shows a variation in which the fenestration space is reduced. The prepontine cistern is the ventral limit of the 3VF and contains the basilar artery. The prepontine interval has been described as an important consideration at the time of surgery.[
Variable features of the 3VF
In addition, other variable features of the 3VF were found.
Separate mammillary bodies
Separate mammillary bodies constitute a challenge for the surgeon as they can be mistaken for the tuber cinereum. This may lead to hypothalamic damage at the time of fenestration [
The prominent basilar artery
The prominent basilar artery occurs in the middle of the tuber cinereum and its herniation, among some communicating vessels, reduces space for fenestration [
Elongated third ventricle
In this type of variant, the anteroposterior diameter of the ventricular cavity is elongated. This anomaly has been described in patients with hydrocephalus.[
Interthalamic bands
Dystopic interthalamic communications have been found that do not pose additional difficulty to the procedure. During the review of intraoperative videos, two new variants were found that have not been described in the literature to date [
Translucent floor: This variant was observed in one patient [
Y-floor: In this variant, there is an increased intermamillary distance and a small prepontine space, with abnormal vasculature that can lead to vascular accidents if there is insufficient experience [
CONCLUSION
Neurosurgeons should recognize the variations of 3VF. Many have been described and this study shows the most common and two new and rare ones. Further studies are needed to determine the relationship between the variations and the operative outcome of patients, as well as to predict the effectiveness of endoscopic fenestration.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anik I, Ceylan S, Koc K, Tugasaygi M, Sirin G, Gazioglu N. Microsurgical and endoscopic anatomy of Liliequist’s membrane and the prepontine membranes: Cadaveric study and clinical implications. Acta Neurochir (Wien). 2011. 153: 1701-11
2. Aydin S, Yilmazlar S, Aker S, Korfali E. Anatomy of the floor of the third ventricle in relation to endoscopic ventriculostomy. Clin Anat. 2009. 22: 916-24
3. Corrales M, Torrealba G. The third ventricle. Normal anatomy and changes in some pathological conditions. Neuroradiology. 1976. 11: 271-7
4. Demerdash A, Rocque BG, Johnston J, Rozzelle CJ, Yalcin B, Oskouian R. Endoscopic third ventriculostomy: A historical review. Br J Neurosurg. 2017. 31: 28-32
5. Dezena RA.editors. Atlas of Endoscopic Neurosurgery of the Third Ventricle. Berlin, Germany: Springer International Publishing; 2017. p.
6. Fabiano AJ, Leonardo J, Grand W. Posterior cerebral artery P1 segment at the stoma during endoscopic third ventriculostomy in adults. J Neurol Neurosurg Psychiatry. 2010. 81: 374-8
7. Giotta Lucifero A, Baldoncini M, Bruno N, Tartaglia N, Ambrosi A, Marseglia GL. Microsurgical neurovascular anatomy of the brain: The anterior circulation (Part I). Acta Biomed. 2021. 92: e2021412
8. Giotta Lucifero A, Baldoncini M, Bruno N, Tartaglia N, Ambrosi A, Marseglia GL. Microsurgical neurovascular anatomy of the brain: The posterior circulation (Part II). Acta Biomed. 2021. 92: e2021413
9. Giotta Lucifero A, Fernandez-Miranda JC, Nunez M, Bruno N, Tartaglia N, Ambrosi A. The modular concept in skull base surgery: Anatomical basis of the median, Paramedian and lateral corridors. Acta Biomed. 2021. 92: e2021411
10. Guzman R, Pendharkar AV, Zerah M, Sainte-Rose C. Use of the NeuroBalloon catheter for endoscopic third ventriculostomy. J Neurosurg Pediatr. 2013. 11: 302-6
11. Hellwig D, Grotenhuis JA, Tirakotai W, Riegel T, Schulte DM, Bauer BL. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev. 2005. 28: 1-34
12. Iaccarino C, Tedeschi E, Rapanà A, Massarelli I, Belfiore G, Quarantelli M. Is the distance between mammillary bodies predictive of a thickened third ventricle floor?. J Neurosurg. 2009. 110: 852-7
13. Jallo GI, Kothbauer KF, Abbott IR. Endoscopic third ventriculostomy. Neurosurg Focus. 2005. 19: E11
14. Kombogiorgas D, Sgouros S. Assessment of the influence of operative factors in the success of endoscopic third ventriculostomy in children. Childs Nerv Syst. 2006. 22: 1256-62
15. Luzzi S, Maestro MD, Elia A, Vincitorio F, Perna GD, Zenga F. Morphometric and radiomorphometric study of the correlation between the foramen magnum region and the anterior and posterolateral approaches to ventral intradural lesions. Turk Neurosurg. 2019. 29: 875-86
16. Resch KD, Perneczky A, Tschabitscher M, Kindel S. Endoscopic anatomy of the ventricles. Acta Neurochir Suppl. 1994. 61: 57-61
17. Rhoton AL. The lateral and third ventricles. Neurosurgery. 2002. 51: S207-71
18. Rohde V, Gilsbach JM. Anomalies and variants of the endoscopic anatomy for third ventriculostomy. Minim Invasive Neurosurg. 2000. 43: 111-7
19. Rohde V, Krombach GA, Struffert T, Gilsbach JM. Virtual MRI endoscopy: Detection of anomalies of the ventricular anatomy and its possible role as a presurgical planning tool for endoscopic third ventriculostomy. Acta Neurochir (Wien). 2001. 143: 1085-91
20. Souweidane MM, Morgenstern PF, Kang S, Tsiouris AJ, Roth J. Endoscopic third ventriculostomy in patients with a diminished prepontine interval. J Neurosurg Pediatr. 2010. 5: 250-4
21. Sughrue ME, Chiou J, Burks JD, Bonney PA, Teo C. Anatomic variations of the floor of the third ventricle: An endoscopic study. World Neurosurg. 2016. 90: 211-27
22. van Aalst J, Beuls EA, van Nie FA, Vles JS, Cornips EM. Acute distortion of the anatomy of the third ventricle during third ventriculostomy. Report of four cases. J Neurosurg. 2002. 96: 597-9