- Department of Neurosurgery, The University of Jordan, Amman, Jordan.
- Department of Special Surgery, School of Medicine, The University of Jordan, Amman, Jordan.
- Department of Pathology, Microbiology and Forensic Medicine, The University of Jordan, Amman, Jordan.
Correspondence Address:
Abdulrahman Al-Shudifat
Department of Pathology, Microbiology and Forensic Medicine, The University of Jordan, Amman, Jordan.
DOI:10.25259/SNI_464_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulrahman Al-Shudifat1, Baraa Mafrachi2, Abdallah Al-Ani2, Amer Khalil Al Qaisi1, Nadwa Bustami3. Ancient Schwannoma affecting the intracranial portion of the trigeminal nerve: A case report. 11-Dec-2020;11:426
How to cite this URL: Abdulrahman Al-Shudifat1, Baraa Mafrachi2, Abdallah Al-Ani2, Amer Khalil Al Qaisi1, Nadwa Bustami3. Ancient Schwannoma affecting the intracranial portion of the trigeminal nerve: A case report. 11-Dec-2020;11:426. Available from: https://surgicalneurologyint.com/surgicalint-articles/10450/
Abstract
Background: Ancient trigeminal schwannomas are extremely uncommon benign tumors. Such tumors are longstanding, slow growing and may demonstrate seemingly malignant features irrespective of its benign nature. The tumor may involve the trigeminal nerve root, the trigeminal ganglion, or any of its peripheral branches. Its clinical presentation may include trigeminal neuralgia, blurry vision, diplopia, or even seizures. Surgical excision is the mainstay of treatment with definite diagnosis only by histopathology.
Case Description: We described a case of a 35-year-old female presenting with recurrent episodes of generalized seizure and left-sided weakness. Brain imaging showed a right temporal space occupying lesion. Results of histopathology were consistent with trigeminal schwannoma associated with ancient histopathological changes. Complete tumor excision was achieved by a two-stage craniotomy, which led to the patient’s condition to dramatically improve.
Conclusion: Ancient trigeminal schwannomas are easily diagnosed through histopathology and result in favorable clinical outcomes after total microscopic surgical excision. A high suspicion index of ancient schwannoma diagnosis should be derived from the patient’s presenting clinical picture and the classical findings derived from neuroimaging.
Keywords: Ancient schwannoma, Intracranial tumor, Trigeminal nerve
INTRODUCTION
Schwannomas are nerve sheath tumors which develop from Schwan cells and are usually benign. Trigeminal schwannomas are a rare entity, accounting for 0.8–5% of intracranial schwannomas,[
CASE PRESENTATION
History and physical examination
A 35-year-old right-handed, medically free female presented to our neurosurgery outpatient clinic complaining of recurrent generalized seizures, chronic facial pain, diffuse dull headache, blurry vision, and dizziness. On physical examination, the patient was confused, scored 14 out of 15 on the Glasgow Coma Scale. She demonstrated a spastic gait, hyperreflexia, and hemiparesis exclusively on her left side. In addition, she scored four out five on the Medical Research Council power scale. Moreover, the patient showed facial asymmetry secondary to a jaw-related pathology, positive Hoffman, Babinski, and Barre’s signs.
Neuroradiology
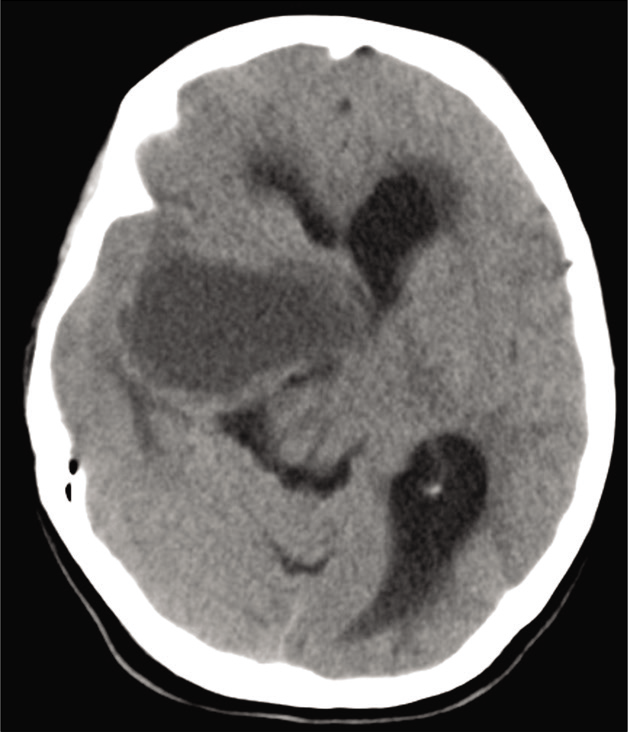
Preoperative brain computer tomography (CT) scan demonstrated a cystic lesion at the right middle fossa on base of the skull [
Figure 1:
Preoperative noncontrasted brain computer tomography-scan demonstrated a hypodense cystic lesion on the right middle cranial fossa at the base of the skull. The lesion extends upward contributing to a mass effect on the contralateral hemisphere. Notice the contralateral ventricular dilatation.
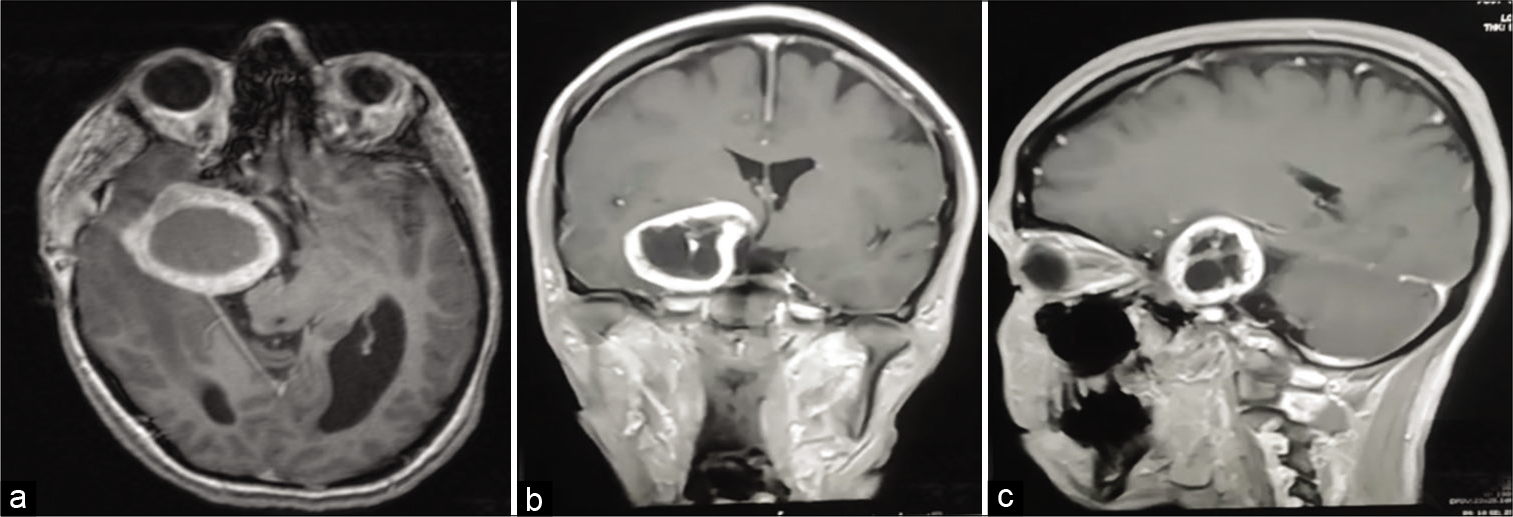
Figure 2:
Three views T1 ([a] Axial, [b] Coronal, [c] Sagittal) Contrasted magnetic resonance imaging showing an extra-axial cystic lesion attached to the skull at the right cavernous sinus associated with significant mass-effect, compression of the brain parenchyma, vasogenic edema in the right temporal and parietal lobes, ventricular compression and subfalcine herniation with active hydrocephalus particularly in the left lateral ventricle. The tumor exhibits a close relation to the right Meckel’s cave and compresses it.
Management
Patient underwent a two-stage right modified pterional craniotomy, with posterior extension, using a sub-temporal approach. Complete excision was achieved under microscope and navigation guidance. The first stage involved the evacuation of the aforementioned cystic component, which was sent for histopathological examination. Subsequently, a complete tumor excision was achieved in the second stage. Intraoperatively and under microscopic visualization, the third division of the trigeminal nerve was indistinguishable from tumor. Nonetheless, the third division was partially preserved through the operation.
Intraoperative neurophysiologic monitoring (IONM) was not utilized due to a myriad of reasons including but not limited to: lack of a standardized IONM procedures,[
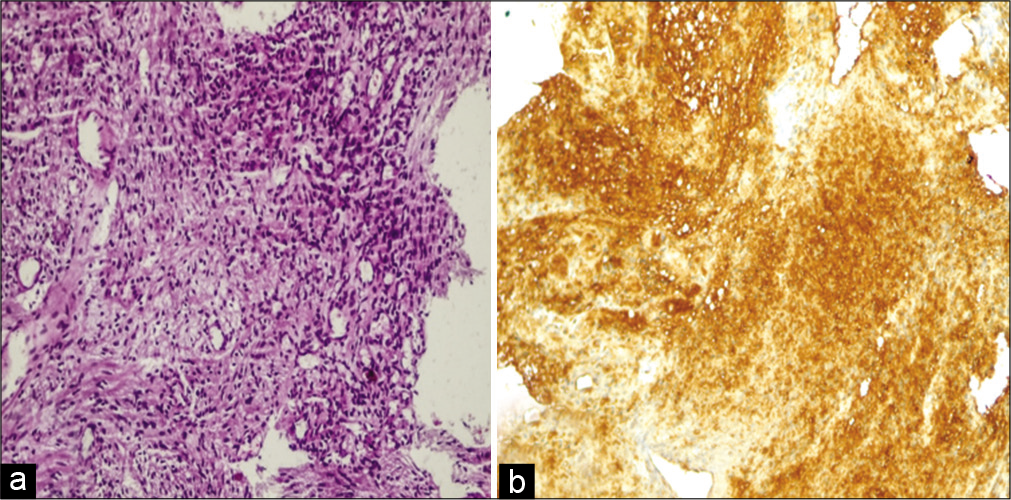
Histopathological examination of the specimen showed a partially encapsulated tumor, composed of bland looking spindle cells that were arranged in hypocellular and hypercellular areas. Nuclear palisading was present in the hypercellular areas. Minimal nuclear atypia was present but no signs of active mitosis were seen. Moreover, thick walled vessels and evidence of previous hemorrhage were evident. The tumor cells were strongly positive for S100 immunostain (both nuclear and cytoplasmic). Both microscopic and immunohistochemial characteristics of the lesion were consistent with ancient schwannoma [
Figure 3:
Histopathological findings. (a) Hematoxylin and Eosin stain, showing spindle cells arranged in fascicles in hypercellular and hypocellular areas, with mild nuclear atypia without any signs of active mitosis. Thin-walled blood vessels were noted. (b) Immunohistochemical staining revealed diffuse positive reactions of cells toward the presence of the S100 protein.
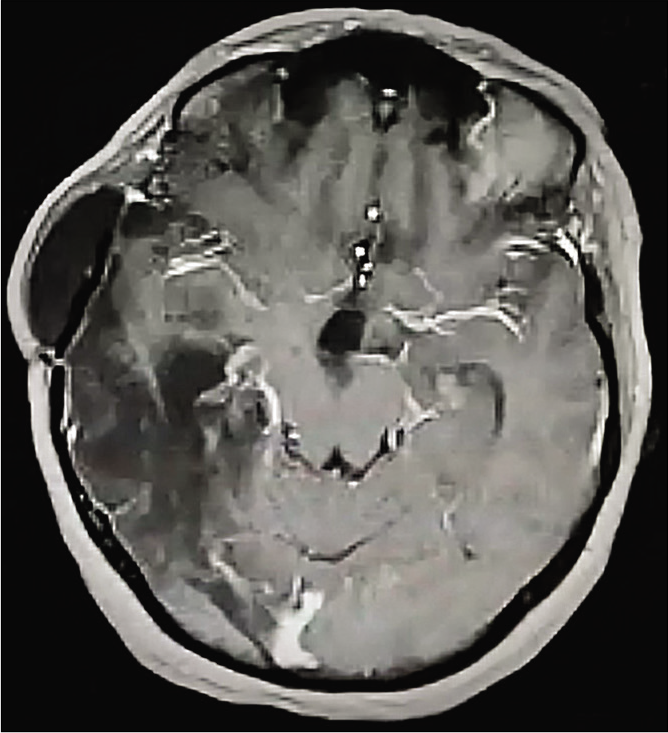
Follow-up
The patient was followed up for 2 years. On her last follow-up, she demonstrated improved symptoms, no power deficit yet complained of the persistence of the aforementioned right-sided mouth deviation. MRI follow-up showed no signs of tumor recurrence [
DISCUSSION
Schwannomas are slow-growing tumors of the perineural Schwan cells. They may involve any myelinated nerve including peripheral, cranial, or autonomic nerves.[
Ancient schwannomas are a benign uncommon histological variant of schwannomas, first described by Ackerman and Taylor.[
Neuroimaging is of paramount importance in establishing the diagnosis, assessing the involvement of adjacent structures, and to determine the best surgical approach. MRI is the imaging modality of choice, in which T1-weighted imaging of trigeminal schwannomas usually shows low signal intensity, while T2-weighted images show high signal intensity. These tumors exhibit momentous enhancement following contrast injection. Brain CT-scan is particularly important for tumors located near the skull base, as these tumors appear as iso- to slightly hypo-dense lesions with variable enhancement postcontrast injection.[
Complete tumor resection with nerve function preservation, minimal postoperative morbidity and mortality are the present goals of trigeminal schwannoma management. With the advances in microsurgical techniques and skull-base approaches, outcomes have dramatically improved with higher survival rates and lower recurrence rates. However, complete tumor resection could be associated with a variety of complications including meningitis, fistula formation, trigeminal neuralgia, and masseter muscle atrophy.[
CONCLUSION
Ancient trigeminal schwannomas are benign intracranial tumors. Diagnosis can be made by histopathology without difficulty. Total microscopic resection can be achieved, resulting in favorable outcomes. Ambiguous cases of ancient trigeminal schwannoma could be handled through fastidious analysis of the presenting patient’s clinical picture and meticulous evaluation of neuroimaging for classical signs.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ackerman LV, Taylor FH. Neijrogenous tumors within the thorax a clinicopatliologacal eualuataon of forty-eight cases. Cancer. 1951. 4: 669-91
2. Agarwal A. Intracranial trigeminal schwannoma. Neuroradiol J. 2015. 28: 36-41
3. Agrawal A, Bhake A, Meshram N, Nisha C. Ancient schwannoma of the trigeminal nerve mimicking high grade lesion. Iran J Pathol. 2010. 5: 97-9
4. Borges A, Casselman J. Imaging the trigeminal nerve. Eur J Radiol. 2010. 74: 323-40
5. Charalampidis A, Jiang F, Wilson JR, Badhiwala JH, Brodke DS, Fehlings MG. The use of intraoperative neurophysiological monitoring in spine surgery. Glob Spine J. 2020. 10: 104S-14
6. Gundamaneni SK, Singh M, Madhugiri VS, Rathakrishnan RK, Sasidharan GM. Trigeminal schwannoma of the sphenoid sinus--report of a rare entity. Br J Neurosurg. 2014. 28: 281-3
7. Kumaria A, Ingale HA, Robertson IJ, Ashpole RD. Trigeminal schwannoma presenting as a gelastic seizure: No laughing matter. Br J Neurosurg. 2018. p. 1-2
8. Micovic MV, Zivkovic BM, Zivanovic JD, Bascarevic VL, Bogosavljevic V, Rasulic LG. Ancient olfactory schwannoma-case report and literature review. Turk Neurosurg. 2017. 27: 656-61
9. Neves MW, De Aguiar PH, Belsuzarri TA, De Araujo AM, Paganelli SL, Maldaun MV. Microsurgical management of trigeminal schwannoma: Cohort analysis and systematic review. J Neurol Surg B Skull Base. 2019. 80: 264-9
10. Puglisi G, Sciortino T, Rossi M, Leonetti A, Fornia L, Nibali MC. Preserving executive functions in nondominant frontal lobe glioma surgery: An intraoperative tool. J Neurosurg. 2019. 131: 474-80
11. Ugokwe K, Nathoo N, Prayson R, Barnett GH. Trigeminal nerve schwannoma with ancient change. Case report and review of the literature. J Neurosurg. 2005. 102: 1163-5
12. Vega-Zelaya L, Pastor J. Neurophysiological monitoring techniques for the resection of malignant brain tumors located in eloquent cortical areas. Austin J Neurosurg. 2015. 2: 1038
13. Zhang L, Yang Y, Xu S, Wang J, Liu Y, Zhu S. Trigeminal schwannomas: A report of 42 cases and review of the relevant surgical approaches. Clin Neurol Neurosurg. 2009. 111: 261-9