- Department of Neurosurgery, Santa Casa de Misericórdia de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil
- Department of Neurosurgery, Hospital Mater Dei, Belo Horizonte, Minas Gerais, Brazil
- Faculdade de Ciências Médicas de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
- Department of Neurosurgery, Hospital das Clínicas da Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Correspondence Address:
Warley Carvalho da Silva Martins
Department of Neurosurgery, Santa Casa de Misericórdia de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil
Department of Neurosurgery, Hospital Mater Dei, Belo Horizonte, Minas Gerais, Brazil
Faculdade de Ciências Médicas de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
DOI:10.4103/sni.sni_109_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marcos Dellaretti, Warley Carvalho da Silva Martins, Jules Carlos Dourado, Wilson Faglioni, Ricardo Souza Quadros, Vítor Vieira de Souza Moraes, Carlos Batista Alves de Souza Filho. Angiographic and epidemiological characteristics associated with aneurysm remnants after microsurgical clipping. 22-Aug-2017;8:198
How to cite this URL: Marcos Dellaretti, Warley Carvalho da Silva Martins, Jules Carlos Dourado, Wilson Faglioni, Ricardo Souza Quadros, Vítor Vieira de Souza Moraes, Carlos Batista Alves de Souza Filho. Angiographic and epidemiological characteristics associated with aneurysm remnants after microsurgical clipping. 22-Aug-2017;8:198. Available from: http://surgicalneurologyint.com/surgicalint-articles/angiographic-and-epidemiological-characteristics-associated-with-aneurysm-remnants-after-microsurgical-clipping/
Abstract
Background:Despite new techniques for the treatment of cerebral aneurysms, the percentage of aneurysm remnants after surgical intervention seems to be relatively constant. The objective of this study was to assess angiographic and epidemiological features associated with aneurysm remnants after microsurgical clipping.
Methods:This study was conducted from February 2009 to August 2012 on a series of 90 patients with 105 aneurysms referred to the Santa Casa of Belo Horizonte who were surgically treated and angiographically controlled.
Results:Surgical clipping was considered incomplete in 13.3% of the aneurysms. The mean age of cases with an aneurysm remnant was 57.5 years, whereas the mean age without aneurysm remnant was 49.7 years (P = 0.02). Aneurysm remnants were detected more frequently on the internal carotid artery, nevertheless, no statistically significant differences were verified when comparing the locations. Aneurysm size in the preoperative angiography verified that the mean size of aneurysms operated was 6.56 mm, such that in cases showing a postoperative remnant, the mean size was 9.7 mm and in cases with complete clipping it was 6.08 mm (P = 0.02). Postoperative angiography showed that, in cases with residual aneurysm, the number of clips used was higher – a mean of 1.8 for complete clipping and 3.1 for incomplete clipping (P
Conclusions:Aneurysm size and patient age showed significant correlations with residual intracranial aneurysm. The mean number of clips used was higher in cases with incomplete occlusion.
Keywords: Aneurysm remnants, angiographic features, epidemiological characteristics, microsurgical clipping
INTRODUCTION
Despite new techniques for the treatment of cerebral aneurysms, such as intraoperative indocyanine green videoangiography and intraoperative micro-Doppler examination, the percentage of aneurysm remnants after surgical intervention seems to be relatively constant. Residual aneurysms are identified in 4–19% of surgical procedures.[
Thus, identifying factors that can predict the presence of aneurysm remnants could contribute toward treatment planning of these patients. The purpose of this study was to assess angiographic and epidemiological features associated with aneurysm remnants after microsurgical clipping.
MATERIALS AND METHODS
Patients
This study was conducted from February 2009 to August 2012 on a series of 90 patients referred to the Santa Casa de Belo Horizonte who were surgically treated and angiographically controlled. Because several patients had multiple aneurysms, 105 aneurysms were clipped and angiographically controlled. The clips applied were mainly Vicca clips (Vicca, Porto Alegre, Rio Grande do Sul, Brazil) made of a titanium alloy.
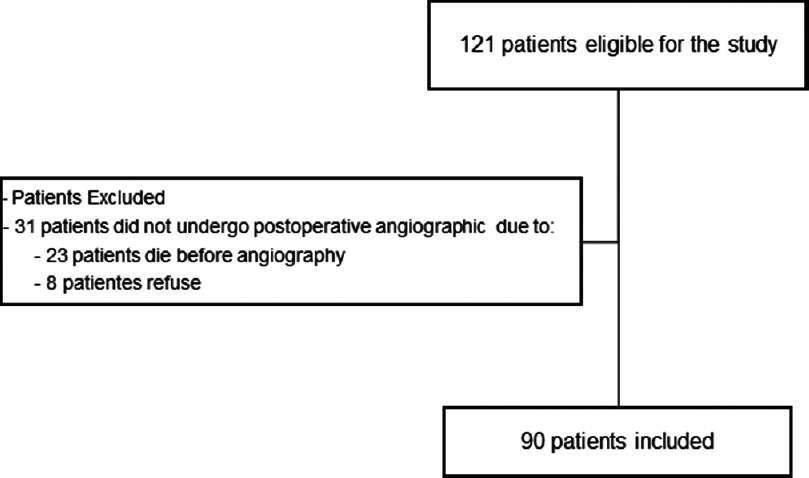
During the study period, 31 additional patients were operated, but did not undergo angiographic follow-up. The reasons for not performing the control examination were death in the postoperative period secondary to severe vasospasm in 23 cases (9.3% of the total series) and patient refusal in 8 cases [
Angiographic study
In the pre- and postoperative periods, patients were submitted to rotational angiography performed using the transfemoral arterial approach in the Neuroradiology Department of our institution followed by three-dimensional (3D) reconstruction of the imaging data.
Angiographic examinations were performed using a biplanar angiographic (unit Axiom Artis dBA, Siemens AG Medical Solutions, Vacuum Technology Division, Henkestr. 127 91052 Erlangen Germany with an image intensifier matrix of 1024 × 1024). In performing digital subtraction angiography (DSA), we tried to optimize the angle to define the clip/aneurysm relationships as well as possible. Usually, we referenced the angle from rotational angiography.
During rotational angiography, the C arm rotated over a 240° range at a speed of 55° per second for about 4.4 seconds. Contrast medium (Iomeron, 300 mg of iodine per mL) was injected at a flow rate of 4–5 mL per second, resulting in total injected volumes of 16–20 mL for each rotational angiographic session in the internal carotid artery (ICA). A flow rate of 2.5–3.5 mL per second resulted in total injected volumes of 10–14 mL into the vertebral artery. Sometimes, a 1-second exposure delay was applied to allow an aneurysm to be fully filled. The image data were transferred to a workstation (Syngo Workplace VA72B, X-Leonardo, Siemens) to reconstruct the 3D images (volume VB35E version software). We analyzed snapshots which consisted of six basic views (anterior, posterior, both lateral, superior, and inferior views) and views from arbitrary angles, as well as cutting views to visualize the clip aneurysm configurations well.
Analysis of residual aneurysms
Radiologically, a residual aneurysm, aneurysm remnant, or incomplete clipping was defined as a small segment at the base of the aneurysm proximal to a clip that was still filled with contrast material in the postoperative angiography.
Aneurysm remnants were classified into 5 grades – grade I, less than 50% of neck size; grade II, more than 50% of neck size; grade III, residual lobe of a multilobulated sac; grade IV, residual portion of the sac less than 75% of aneurysm size; and grade V, residual portion of the sac more than 75% of aneurysm size.[
Data analysis
The variables studied were aneurysm size, neck size, and location, determined by preoperative angiography; number of clips, determined by postoperative angiography; and patient age. The endpoint was the presence of residual aneurysm after microsurgical clipping identified by postoperative angiography.
Medcalc software was used for data analysis. For variables in which the mean was evaluated, such as patient age, aneurysm size, neck size, and number of clips, analysis of variance (ANOVA) test was used. The Chi-square test and Chi-square trend test were used when evaluating categorical variables, such as aneurysm location, and aneurysm size, classified as small (less than 7 mm), medium (7–15 mm), large or giant (larger than 15 mm). In cases in which the value of one of the variables was lower than 5, the Fisher test was used. P values were considered statistically significant when less than 0.05.
RESULTS
Of the 90 patients treated and submitted to both pre- and postoperative angiography examinations, 66 were females and 24 were males. Patient age ranged from 26 to 80 years, with a mean of 50.7 years.
Surgical clipping was considered incomplete in 14 aneurysms (13.3%). All were cases with remnants less than 50% of the neck size (grade I), none of the patients were submitted to reoperation [Figures
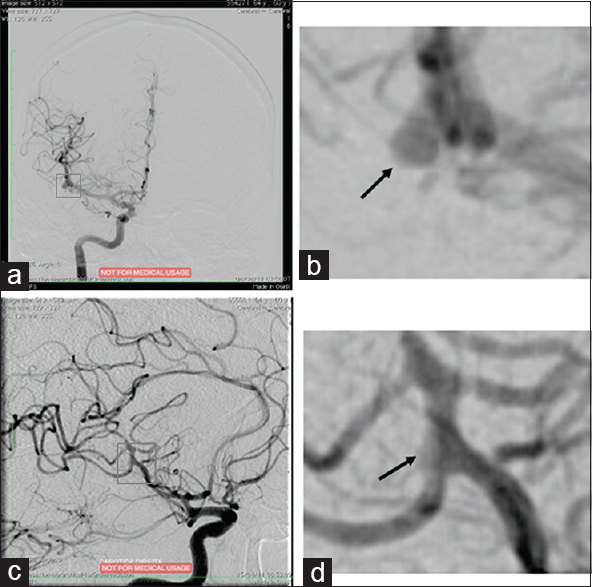
Figure 2
Arteriography of a 60-year-old man with headaches and meningism. (a) Right middle cerebral artery sacular aneurysm, measuring 7.1 × 3.2 mm; (b) The black arrow indicates the aneurysm; (c) Aneurysm clipping performed with 2 clips. Identified an aneurysm remnant, which was treated conservatively; (d) The black arrow indicates the remnant aneurysm
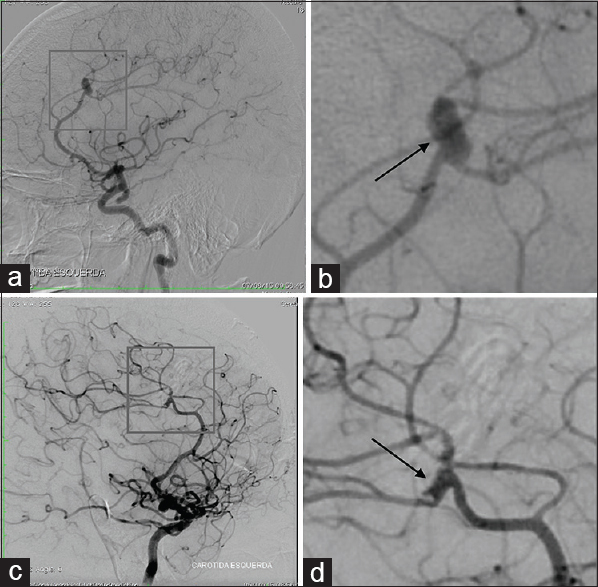
Figure 3
Arteriography of a 52-year-old woman with an incidental aneurysm. (a) Left pericallosal artery saccular aneurysm, measuring 6.0 × 2.5 mm; (b) The black arrow indicates the aneurysm; (c) Aneurysm clipping performed with 3 clips. An aneurysm remnant measuring 1.1 mm was identified and treated conservatively; (d) The black arrow indicates the remnant aneurysm
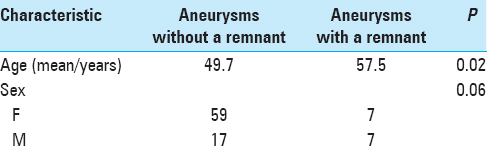
The mean patient age of cases with an aneurysm remnant was 57.5 years, whereas the mean patient age without aneurysm remnant was 49.7 years; this difference was statistically significant (P = 0.02) [
Aneurysm location
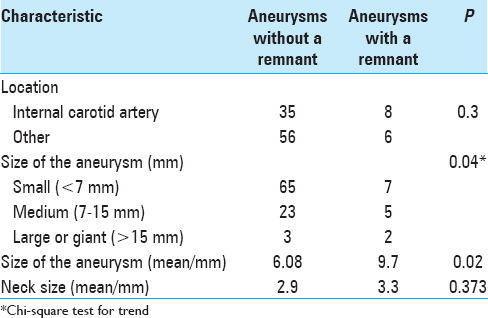
Regarding the location of the 105 operated aneurysms, 100 (95.2%) were located in the anterior circulation and 5 (4.8%) in the posterior circulation. All the aneurysms that showed remnants were in the anterior circulation, 14.0% of these cases. Eight of the 14 aneurysm remnants were located on the ICA, 3 on the ophthalmic segment, 4 on the posterior communicating artery (PCoA), and 1 on the anterior choroidal artery (AChoA); 2 were on the middle cerebral artery (MCA) and 4 were on the anterior communicating artery (ACoA). Although aneurysm remnants were detected more frequently on the ICA, no statistically significant differences were noted regarding location [
Aneurysm size
Regarding the size of the aneurysms, 72 were small (<7 mm), and of these 7 (9.7%) showed an aneurysm remnant in the postoperative arteriography. Twenty-eight aneurysms were medium (7–15 mm) of these 5 (17.8%) showed an aneurysm remnant. Only 5 aneurysms were large or giant (>15 mm), and of these 2 (40.0%) showed an aneurysm remnant in the postoperative arteriography [
Evaluation of aneurysm size in the preoperative angiography verified that the mean size of aneurysms operated was 6.56 mm, such that in cases showing a postoperative remnant, the mean size was 9.7 mm, and in cases with complete clipping it was 6.08 mm; this difference was statistically significant (P = 0.02). Similarly, regarding aneurysm neck size, for aneurysms showing a remnant, the mean size was 3.3 mm, and for aneurysms without a remnant the mean was 2.9 mm. Despite the larger neck size in aneurysms showing a postoperative remnant, this difference was not statistically significant (P = 0.373) [
Number of clips required
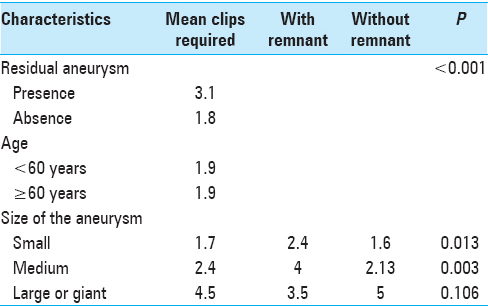
Postoperative angiography also showed that, in cases with residual aneurysm, the number of clips used was higher: a mean of 1.8 for complete clipping and 3.1 for incomplete clipping (P < 0.001) [
Comparison of the number of clips used with patient age showed that the mean number of clips used for an aneurysm in patients less than 60 years and patients 60 years of age or older was 1.9 clips in both groups [
Comparison of the number of clips required with aneurysm size showed that a mean of 1.7 clips were used for small aneurysms, whereas for small aneurysms showing a remnant, the mean was 2.4 clips, and for small aneurysms without a remnant it was 1.6 clips (P = 0.013). For medium-sized aneurysms, the mean number of clips used for each aneurysm was 2.4, whereas for medium-sized aneurysms without a remnant, the mean was 2.13, and in medium-sized aneurysms showing a remnant it was 4 clips (P = 0.003). For large or giant aneurysms, the mean number of clips used for each aneurysm was 4.5, whereas for large or giant aneurysms without a remnant, the mean was 5 clips, and in large or giant aneurysms showing a remnant it was 3.5 clips (P = 0.106) [
DISCUSSION
The importance of identifying residual aneurysms is due to the risk of hemorrhage from these remnants.[
In a retrospective study involving postoperative arteriography, Le Roux et al.[
Although some authors have reported no relationship between aneurysm size and the presence of remnants,[
Aneurysm size and mean aneurysm size were also predictors of residual aneurysm in this study. The rate of residual aneurysm was 9.7% for small aneurysms and 40.0% for large or giant aneurysms. Furthermore, the mean size of aneurysms measured in the preoperative arteriography was 9.7 mm in cases that showed a remnant and 6.6 mm in cases with no remnant. Some aneurysm remnants were left intentionally due to the complexity of the operation during clipping and the risk of vessel stenosis induced by a complete clipping. This decision was based on the current understanding that grade 1 remnants present low rates of rebleeding.
In this study, even though aneurysm remnants were observed more frequently in the ICA, no statistically significant differences were observed compared with other locations. Regarding these findings, the literature is controversial. In a study involving intraoperative arteriography, Derdeyn et al.[
The number of clips required for aneurysm exclusion was shown to be another predisposing factor for the presence of residual aneurysm. Moreover, the number of clips used in small and medium-sized aneurysms in cases where a remnant was observed was significantly higher than in cases without a remnant. Although the literature has not established an association between this variable and any increase in residual aneurysm, multiple clip applications during surgery was identified as a predictor of residual aneurysm by Le Roux et al.[
Jabbarli et al.[
Finally, even though the risk of hemorrhage was present, in this study, none of the patients with aneurysm remnants were submitted to reoperation. This approach is similar to other authors, such as Sindou et al.,[
However, a risk of rupture per year makes the cumulative risk one of importance, particularly in young patients.[
Intraoperative indocyanine green videoangiography and intraoperative angiography are methods used to avoid aneurysm remnants. Intraoperative indocyanine green videoangiography has the advantage of easy-to-perform intraoperative method for evaluating the occluded dome or neck of a cerebral aneurysm during surgery to avoid residual aneurysms. Thus, the very low complication rate, low costs of the technique, and described benefit rate of nearly 15% of the procedures argue for routine application during clipping of cerebral aneurysms. In contrast, in up to 10% of patients, small aneurysm sac remnants can be overlooked intraoperatively by indocyanine green videoangiography.[
Despite the limitations of this study, including its retrospective view and the lack of an evaluation of morphological features that can be associated with aneurysm remnants, the findings and suggestions proposed can contribute to improving presurgical planning. Our analysis demonstrates that an incomplete occlusion is more likely in older patients, and/or in patients with larger or giant aneurysms.
CONCLUSIONS
Aneurysm size and patient's age showed significant correlations with residual intracranial aneurysm. The mean number of clips used was higher in cases with incomplete obstruction probably due to the complex anatomy of these aneurysms.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Financial support and sponsorship
Nil.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
1. Alexander TD, Macdonald RL, Weir B, Kowalczuk A. Intraoperative angiography in cerebral aneurysm surgery: A prospective study of 100 craniotomies. Neurosurgery. 1996. 39: 10-7
2. David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999. 91: 396-401
3. Derdeyn CP, Moran CJ, Cross DT, Sherburn EW, Dacey RG. Intracranial aneurysm: Anatomic factors that predict the usefulness of intraoperative angiography. Radiology. 1997. 205: 335-9
4. Drake CG, Friedman AH, Peerless SJ. Failed aneurysm surgery. Reoperation in 115 cases. J Neurosurg. 1984. 61: 848-56
5. Drake CG, Vanderlinden RG. The Late Consequences of Incomplete Surgical Treatment of Cerebral Aneurysms. J Neurosurg. 1967. 27: 226-38
6. Feuerberg I, Lindquist C, Lindqvist M, Steiner L. Natural-history of postoperative aneurysm rests. J Neurosurg. 1987. 66: 30-4
7. Jabbarli R, Pierscianek D, Wrede K, Dammann P, Schlamann M, Forsting M. Aneurysm remnant after clipping: The risks and consequences. J Neurosurg. 2016. 125: 1249-55
8. Kang HS, Han MH, Kwon BJ, Jung SI, Oh CW, Han DH. Postoperative 3D angiography in intracranial aneurysms. Am J Neuroradiol. 2004. 25: 1463-9
9. Lawton MT.editorsSeven Aneurysms. Tenets and techniques for clipping. New York: Thieme; 2011. p.
10. Le Roux PD, Elliott JP, Eskridge JM, Cohen W, Winn HR. Risks and benefits of diagnostic angiography after aneurysm surgery: A retrospective analysis of 597 studies. Neurosurgery. 1998. 42: 1248-54
11. Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg. 1989. 70: 556-60
12. Macdonald RL, Wallace MC, Kestle JRW. Role of angiography following aneurysm surgery. J Neurosurg. 1993. 79: 826-32
13. Park CK, Shin HS, Choi SK, Lee SH, Koh JS. Clinical analysis and surgical considerations of atherosclerotic cerebral aneurysms: Experience of a single center. J Cerebrovasc Endovasc Neurosurg. 2014. 16: 247-53
14. Payner TD, Horner TG, Leipzig TJ, Scott JA, Gilmor RL, DeNardo AJ. Role of Intraoperative Angiography in The Surgical Treatment of Cerebral Aneurysms. J Neurosurg. 1998. 88: 441-8
15. Roessler K, Krawagna M, Dörfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: Conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 2014. 36: E7-
16. Sakaki T, Takeshima T, Tominaga M, Hashimoto H, Kawaguchi S. Recurrence of ICA-PCoA aneurysms after neck clipping. J Neurosurg. 1994. 80: 58-63
17. Sato S, Suzuki J. Prognosis in cases of intracranial aneurysm after incomplete direct operations. Acta Neurochir. 1971. 24: 245-52
18. Sindou M, Acevedo CJ, Turjman F. Aneurysmal Remnants After Microsurgical Clipping: Classification and Results from a Prospective Angiographic Study (in a Consecutive Series of 305 Operated Intracranial Aneurysms). Acta Neurochir. 1998. 140: 1153-9
19. Steven JL. Postoperative angiograph in treatment of intracranial aneurisms. Acta Radiol Diagn. 1966. 5: 536-48
20. Washington CW, Zipfel GJ, Chicoine MR, Derdeyn CP, Rich KM, Moran CJ. Comparing indocyanine green videoangiography to the gold standard of intraoperative digital subtraction angiography used in aneurysm surgery. J Neurosurg. 2013. 118: 420-7