- Department of Neurology, University of Cape Town, Western Cape, South Africa.
Correspondence Address:
Aayesha Soni, Department of Neurology, University of Cape Town, Western Cape, South Africa.
DOI:10.25259/SNI_796_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aayesha Soni, Edward Lee Pan, Lawrence Tucker. Anterior temporal lobectomy: A cross-sectional observational study of potential surgical candidates at a single institute. 16-Nov-2021;12:565
How to cite this URL: Aayesha Soni, Edward Lee Pan, Lawrence Tucker. Anterior temporal lobectomy: A cross-sectional observational study of potential surgical candidates at a single institute. 16-Nov-2021;12:565. Available from: https://surgicalneurologyint.com/surgicalint-articles/11224/
Abstract
Background: Epilepsy is a common neurological disorder, associated with serious cognitive, physical, and psychosocial burdens. Mesial temporal lobe epilepsy (mTLE) is the commonest form of focal epilepsy. The aim of this study was to establish the incidence of patients with electroencephalographic epileptiform discharges consistent with mTLE attending a tertiary hospital in South Africa, and determine whether these patients may be candidates for anterior temporal lobectomy.
Methods: This was a cross-sectional observational study of all patients receiving scalp electroencephalograms (EEG) performed at the Groote Schuur Hospital Neurophysiology laboratory during the period January 1, 2017–December 31, 2019. Where magnetic resonance imaging (MRI) brain scans had been performed, these were assessed for corroborative evidence of mTLE.
Results: Over the 3-year period, 4 342 EEGs were assessed. A total of 411 (11%) showed epileptiform discharges consistent with all epilepsy types. Of these, 327 (69%) were of focal onset and 108 (33% of all focal onset epilepsies) were consistent with mTLE. Of the patients with electroencephalographic features of mTLE, only 27 (25%) had had MRI brain scans performed according to an epilepsy surgery protocol. None of these patients had been considered for surgery.
Conclusion: Surgery, especially anterior temporal lobectomy, is widely acknowledged to be an efficacious and cost-effective intervention in patients with drug-resistant mTLE. The findings of our study suggest that patients with mTLE in our setting are under-investigated for potential surgery; and that it is under-utilized. These findings are in line with similar studies in both well-resourced and resource-constrained countries. Our study also highlights the utility of EEG as a practical screening tool to identify potential surgical candidates, as well as the establishment of an EEG and MRI database to assist in recognizing these patients.
Keywords: Anterior temporal lobectomy, Electroencephalogram, Mesial temporal lobe epilepsy
INTRODUCTION
Epilepsy is a common neurological disorder, which is estimated to affect at least 60 million people of all ages worldwide.[
In approximately 60% of patients with epilepsy, seizures have a focal onset.[
The aim of this study was to determine the number of patients with anterior and/or middle temporal lobe epileptiform features on non-invasive EEG consistent with mTLE referred to a South African, public sector tertiary hospital in Cape Town over a 3-year period and determine what proportion of these patients had been appropriately worked-up as potential candidates for epilepsy surgery. We suspected that patients are under-evaluated for surgical intervention; and that epilepsy surgery is under-utilised in our setting.
MATERIALS AND METHODS
Study population
This was a cross-sectional observational study of 4,342 consecutive EEGs performed on patients referred to the Groote Schuur Hospital (GSH) electrophysiological laboratory over a 3-year period (January 1, 2017-December 31, 2019). The study population included all adults (older than 13 years of age), who were referred for a scalp EEG. EEGs of younger persons, all recordings rendered illegible by severe persistent artefacts and duplicate patient EEGs were excluded.
Methods
All EEGs were performed in the neurophysiology laboratory or the wards of GSH. Nihon Kohden 21 channel digital EEG machines and software, and the international 10-20 electrode placement system are used along with 2 additional electrodes: FT9/T1 and FT10/T2 from the 10-10 system. All EEGs were performed by qualified technologists and recordings routinely lasted a minimum of 20 min, unless aborted earlier due to external medical or patient-related circumstances. Duplicate EEGs for the same patient were removed with the most abnormal report being used. Hyperventilation, intermittent photic stimulation, and mental arousal procedures were routinely performed in the electrophysiology laboratory. All EEGs were performed on out-patients and in-hospital patients at GSH, or patients referred from the surrounding secondary and primary healthcare facilities. Reasons for referral included unexplained losses of consciousness, suspected seizures, epilepsy, confusion, encephalopathy, dementia, and psychosis, among others.
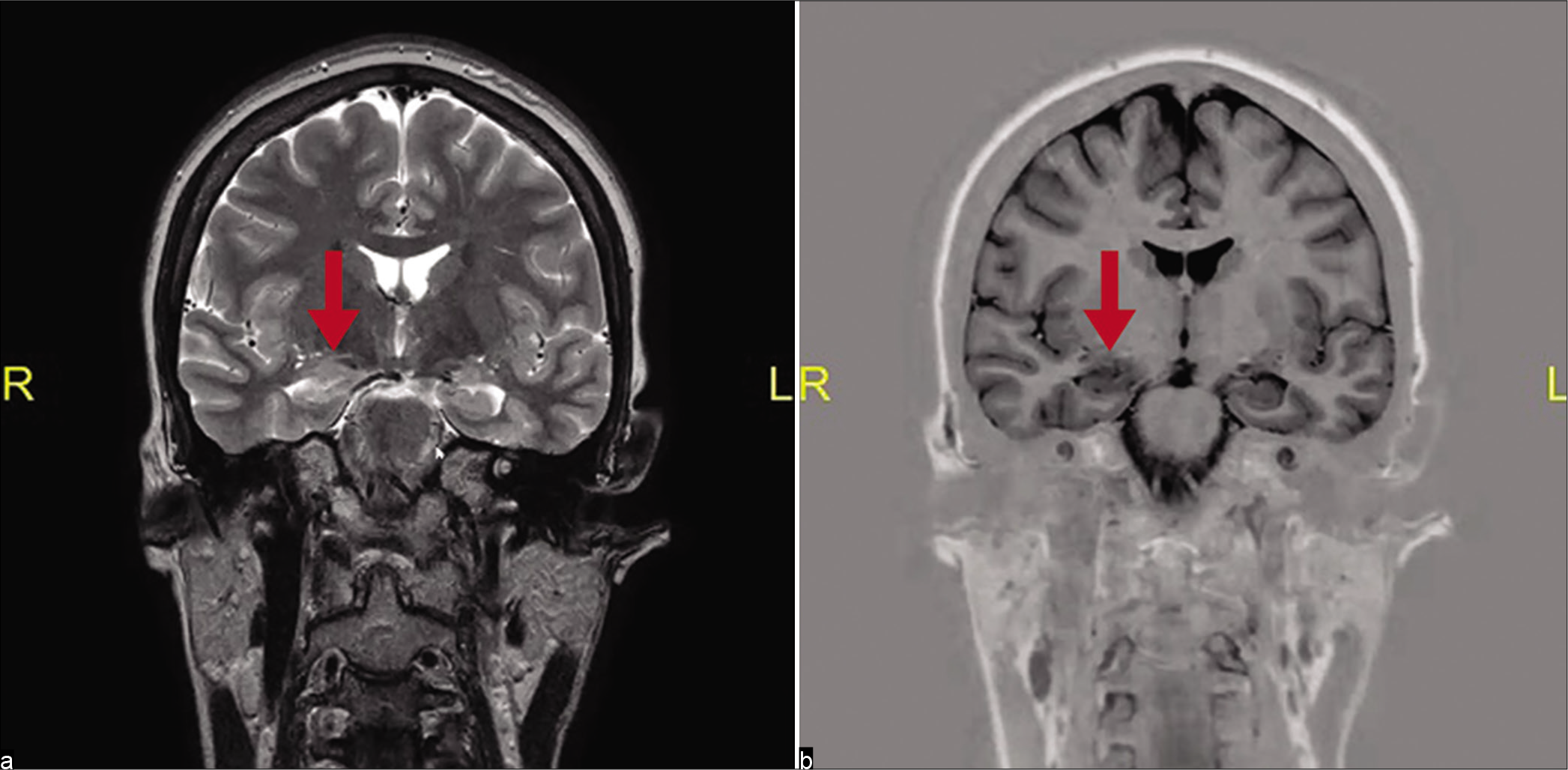
Anonymised patient data relating to EEG and brain MRI findings for this study were recorded on a Microsoft Excel Sheet and stored on a password-protected laptop. All EEGs performed during the study period were accessed and those reported by an attending specialist neurologist to show temporal lobe epileptiform activity were identified. The identified EEG traces were reviewed by a trainee neurologist experienced in EEG interpretation to confirm the presence and location of temporal epileptiform activity. These were then further classified as anterior and/or middle temporal, or posterior temporal lobe discharges. Hospital numbers of patients with anterior and/or middle temporal lobe epileptiform discharges were used on the GSH Picture Archiving and Communication System to identify those patients in whom MRI or computed tomography (CT) brain scanning had been performed, and whether mesial temporal sclerosis had been confirmed on an MRI using an epilepsy surgery protocol, which included axial and coronal T1-weighted, T2-weighted FLAIR, and T1-inversion recovery sequences. A trainee neurologist and specialist neurologist experienced in epilepsy management reviewed images of all MRI brain scans performed on patients with anterior and/or middle temporal epileptiform discharges to confirm radiological evidence of mesial temporal sclerosis and hippocampal atrophy [
Ethics
Ethics clearance was granted by the University of Cape Town’s Human Research Ethics Committee (HREC Reference number: 365/2020).
Statistical analysis
Summary statistics are reported using frequencies and percentages for categorical responses and means with standard deviations for continuous measurement. With regards to inferential statistics, this involved cross-tabulations, ANOVA and correlation analyses which were used as appropriate for the types of variables compared.
RESULTS
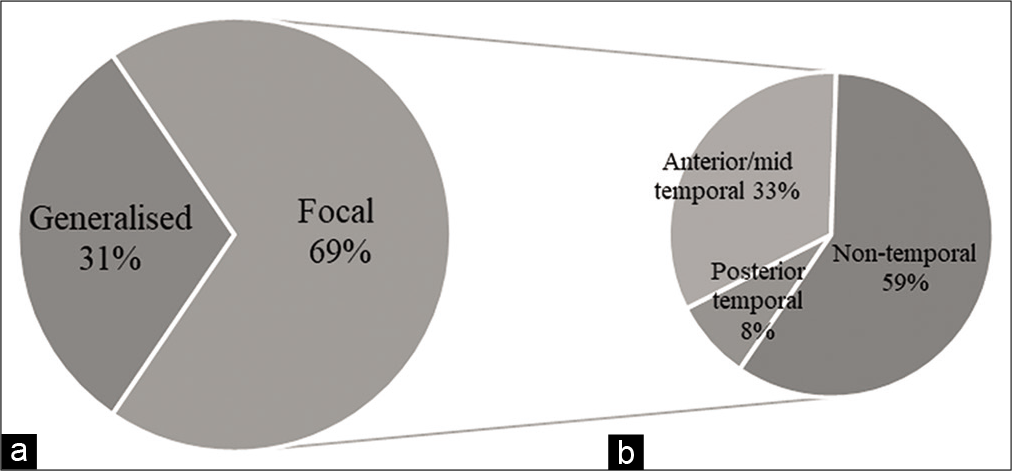
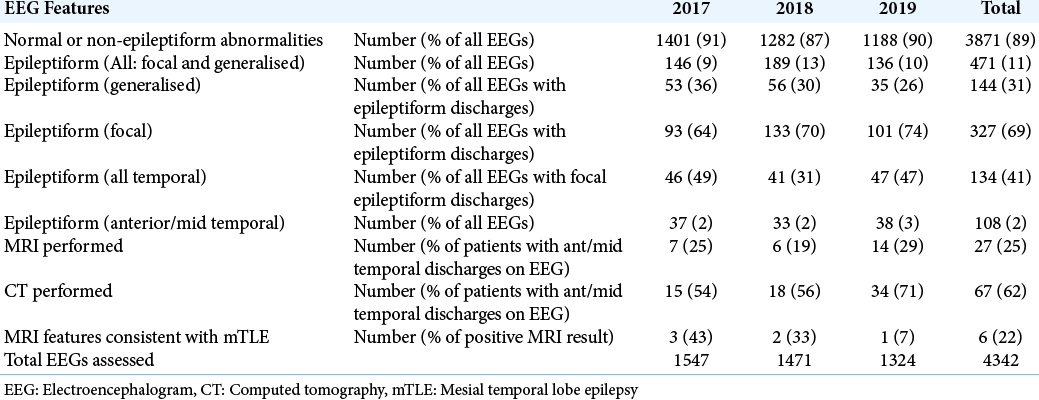
The study sample included all EEGs performed at the GSH Neurophysiology laboratory over a 3-year period between January 1, 2017, and December 31, 2019. A total of 4,342 EEGs were assessed, with 27 EEGs being excluded based on duplication or illegibility. Of these, 411 (11%) showed evidence supporting epilepsy, of which 327 (69%) were focal and 144 (31%) generalised.
Temporal lobe epileptiform discharges were identified in 134 EEGs, which represented 41% of all EEGs with focal epileptiform discharges. Of all EEGs with focal temporal lobe discharges, 108 (81%) were in the anterior and/or middle temporal lobe, and 26 (19%) in the posterior temporal lobe. Thus, anterior and/or middle temporal lobe discharges consistent with mTLE were identified in 108 (2%) of all EEGs, and 33% of EEGs with focal discharges, over the 3-year study period. [
Appropriate MRI of the brain scanning according to an epilepsy surgery protocol had been performed in only 27 (25%) of the 108 patients with anterior and/or middle temporal lobe epileptiform discharges on EEG. Of these, six had MRI evidence of mesial temporal sclerosis ipsilateral to the epileptiform discharge. All six of these patients had been diagnosed with drug-resistant epilepsy and were potential surgical candidates, but none had been subsequently worked up further or considered for epilepsy surgery. [
DISCUSSION
The prevalence of focal epilepsy is reported to be higher in Africa, Latin America and other low- and middle-income regions compared with high-income countries, with rural regions being especially affected.[
mTLE is the focus of this study because anterior temporal lobectomy is a highly effective and cost-efficient intervention in the management of drug-resistant seizures in patients with this condition, frequently rendering carefully chosen subjects seizure-free. Wiebe et al. have demonstrated the superiority of surgery over medical therapy in terms of seizure control, quality of life, and the rates of employment or school attendance in patients with drug-resistant TLE.[
Despite its efficacy and safety, epilepsy surgery remains under-utilised in both low- and high-income countries.[
The long-term costs of drug-resistant epilepsy are well recognised. These include life-long multiple AED administration, recurrent hospital and intensive care admissions for seizure-related injuries and status epilepticus, as well as unemployment and the psycho-social burden on the patient and society. Therefore, the economic benefits of rendering a patient with drug-resistant mTLE seizure-free are well established and accepted.[
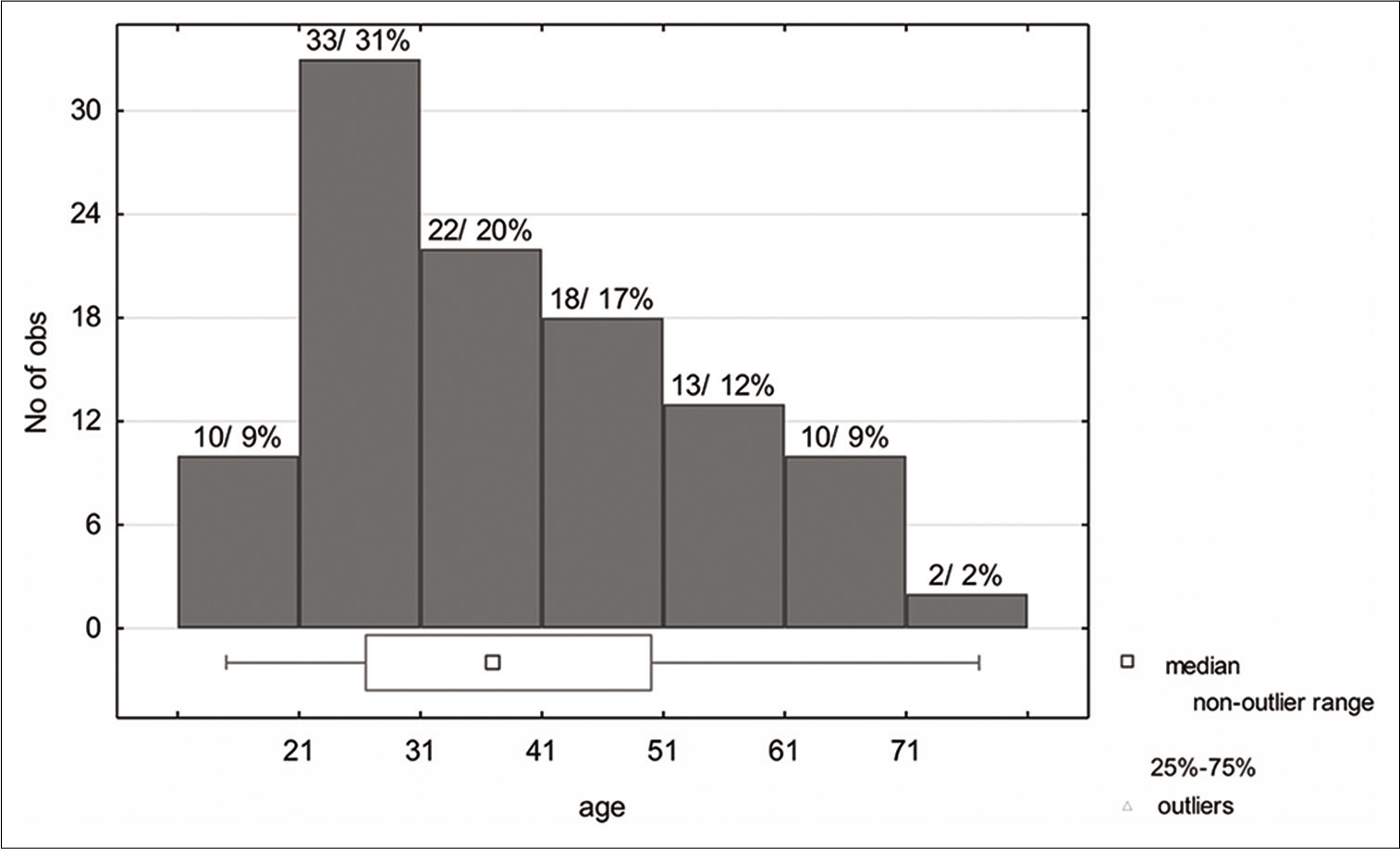
Our study confirmed that most patients with temporal epileptiform discharges identified on EEGs performed at a large South African tertiary hospital were not adequately investigated with MRI brain scanning, long-term monitoring or neuropsychometric assessment to determine whether they were candidates for anterior temporal lobectomy. This is despite the fact that we have the resources and expertise to conduct all of the above, in addition to a competent and willing neurosurgical team to perform the surgery. It is significant that 31% (33) of patients identified with temporal lobe epileptiform activity in this study were between the ages of 21–31 years, and that epilepsy surgery, were it performed, would potentially render them seizure-free for the remainder of their lives.
In 62% of the patients with temporal lobe epileptiform activity, CT brain imaging (with and without contrast) was performed, rather than MRI. CT is a cheaper investigation and while effective in excluding tumors, parasitic infections, granulomas, haemorrhages and many other structural intracranial abnormalities, MRI is a prequisite for epilepsy surgery and the investigation of choice for identifying mesial temporal sclerosis, neuronal migration disorders and other subtle epileptogenic abnormalities which may be amenable to epilepsy surgery. MRI brain scans performed according to a standardised epilepsy surgery protocol were performed in only 25% of the patients with anterior and/or middle temporal lobe epileptiform discharges in our study. To improve the efficacy of identifying candidates for epilepsy surgery, the application of an appropriate MRI protocol is vital. For example, an “essential 6” sequence protocol has recently been suggested for the detection of virtually all common epileptogenic lesion entities.[
Our study found that, over the 3-year study period, 11% (411) of all EEGs showed epileptiform discharges, 69% (327) of which were focal in onset and significantly, a third of EEGs with focal discharges (108) (33%; P = 0.00089) were located in the anterior and/or-middle temporal lobe consistent with mTLE, and thus were potential candidates for anterior temporal lobectomy. A limitation to our study is that a standard 20-min scalp EEG may not always identify interictal epileptiform discharges, and this could mean patients may be missed. Another limitation is that patients were identified on an EEG database where the EEG had already been reported on, and this could have meant that patients with EEGs showing anterior-middle temporal epileptiform discharges could have been missed based on reporter abilities.
The results of this study support the impression that patients with mTLE are under-investigated, and that epilepsy surgery is under-utilised in a tertiary healthcare centre in South African. Consideration should be given to increasing the use of surgery in low- and middle-income countries such as ours, where the expertise of a multi-disciplinary team is available and economic benefits are established. The study also demonstrates that standard scalp EEGs may be an effective way in which to identify potential epilepsy surgery candidates. These EEGs may be used as an initial tool to identify patients who should receive brain MRIs and be further worked up for potential surgery. Still, using non-invasive EEGs as an initial screening tool could offer resource-constrained settings a stepwise protocol that could be implemented in patients with mTLE, in whom safe and cost-effective anterior temporal lobectomies may result in seizure freedom. A database of the epilepsy patients or radiological images of these patients did not exist in our hospital prior to this study, which is another practical implementation that offers resource-constrained settings the platform to identify potential surgical candidates more readily.
CONCLUSION
Surgery in the form of anterior temporal lobectomy is a relatively straightforward, safe, and cost-effective intervention in carefully selected patients with drug-resistant mTLE, and frequently results in seizure freedom. In line with the findings of similar studies in other low-, middle- and high-income countries, our study indicates that appropriate work up of these patients for possible neurosurgical intervention is lacking, and that epilepsy surgery is under-utilised. This is despite the infrastructure and expertise being available to investigate and assess these patients adequately and perform the procedure in some centres, such as ours. Our study also highlights the utility of an EEG as a practical screening tool to identify potential surgical candidates, as well as the benefit of the establishment of an EEG and MRI database to assist in recognising these patients. We encourage these measures to motivate increased surgeries in low- and middle-income countries.
Author contributions
AJ Soni was responsible for data collection and interpretation. EB Lee Pan, AJ Soni and LM Tucker supervised the study and contributed to the preparation and intellectual content of the manuscript.
Declaration of patient consent
Patients’ consent not required as patients’ identities not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barbaro NM, Quigg M, Ward MM, Chang EF, Broshek DK, Langfitt JT. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia. 2018. 59: 1198-207
2. Benbadis SR, Heriaud L, Tatum IV, Vale FL. Epilepsy surgery, delays and referral patterns-are all your epilepsy patients controlled?. Seizure. 2003. 12: 167-70
3. Boling WW. Surgical considerations of intractable mesial temporal lobe epilepsy. Brain Sci. 2018. 8: 35
4. Brotis AG, Giannis T, Kapsalaki E, Dardiotis E, Fountas KN. Complications after anterior temporal lobectomy for medically intractable epilepsy: A systematic review and meta-analysis. Stereotact Funct Neurosurg. 2019. 97: 69-82
5. Butler JT. The role of epilepsy surgery in Southern Africa. Acta Neurol Scand Suppl. 2005. 181: 12-6
6. Engel J. A greater role for surgical treatment of epilepsy: Why and when?. Epilepsy Curr. 2003. 3: 37-40
7. Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012. 307: 922-30
8. Hakimi AS, Spanaki MV, Schuh LA, Smith BJ, Schultz L. A survey of neurologists’ views on epilepsy surgery and medically refractory epilepsy. Epilepsy Behav. 2008. 13: 96-101
9. Hemb M, Palmini A, Paglioli E, Paglioli EB, Costa da Costa J, Azambuja N. An 18-year follow-up of seizure outcome after surgery for temporal lobe epilepsy and hippocampal sclerosis. J Neurol Neurosurg Psychiatry. 2013. 84: 800-5
10. Lee AT, Burke JF, Chunduru P, Molinaro AM, Knowlton R, Chang EF. A historical cohort of temporal lobe surgery for medically refractory epilepsy: A systematic review and meta-analysis to guide future nonrandomized controlled trial studies. J Neurosurg. 2020. 133: 71-8
11. Mac TL, Tran DS, Quet F, Odermatt P, Preux PM, Tan CT. Epidemiology, aetiology, and clinical management of epilepsy in Asia: A systematic review. Lancet Neurol. 2007. 6: 533-43
12. Matsuura M. Indication for anterior temporal lobectomy in patients with temporal lobe epilepsy and psychopathology. Epilepsia. 2000. 41: 39-42
13. Meng Y, Elkaim L, Wang J, Liu J, Alotaibi NM, Ibrahim GM. Social media in epilepsy: A quantitative and qualitative analysis. Epilepsy Behav. 2017. 71: 79-84
14. Oddo S, Solis P, Consalvo D, Seoane E, Giagante B, D’Alessio L. Postoperative neuropsychological outcome in patients with mesial temporal lobe epilepsy in Argentina. Epilepsy Res Treat. 2012. 2012: 370351
15. Picot MC, Jaussent A, Neveu D, Kahane P, Crespel A, Gelisse P. Cost-effectiveness analysis of epilepsy surgery in a controlled cohort of adult patients with intractable partial epilepsy: A 5-year follow-up study. Epilepsia. 2016. 57: 1669-79
16. Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005. 4: 21-31
17. Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B. Is the underlying cause of epilepsy a major prognostic factor for recurrence?. Neurology. 1998. 51: 1258-61
18. Sheikh SR, Kattan MW, Steinmetz M, Singer ME, Udeh BL, Jehi L. Cost effectiveness of surgery for drug resistant temporal lobe epilepsy in the US. Neurology. 2020. 95: e1404-16
19. Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis. Brain. 2005. 128: 1188-98
20. Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012. 2012: 6
21. Wellmer J, Quesada CM, Rothe L, Elger CE, Bien CG, Urbach H. Proposal for a magnetic resonance imaging protocol for the detection of epileptogenic lesions at early outpatient stages. Epliepsia. 2013. 54: 1977-87
22. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomised controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345: 311-8