- Department of Neurosurgery, University of Arizona, Tucson, United States.
- Department of Neurosurgery, University of Arizona College of Medicine, Phoenix, Arizona, United States.
Correspondence Address:
Mauricio J. Avila, Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States.
DOI:10.25259/SNI_837_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mauricio J. Avila1, José Manuel Orenday-Barraza2, María José Cavagnaro1, Isabel M. Strouse2, Dara S. Farhadi2, Naushaba Khan2, Amna Hussein2, Ali A. Baaj2. Antifibrinolytics use during surgery for oncological spine diseases: A systematic review. 02-Dec-2022;13:567
How to cite this URL: Mauricio J. Avila1, José Manuel Orenday-Barraza2, María José Cavagnaro1, Isabel M. Strouse2, Dara S. Farhadi2, Naushaba Khan2, Amna Hussein2, Ali A. Baaj2. Antifibrinolytics use during surgery for oncological spine diseases: A systematic review. 02-Dec-2022;13:567. Available from: https://surgicalneurologyint.com/surgicalint-articles/12034/
Abstract
Background: Data exist of the benefits of antifibrinolytics such as tranexamic acid (TXA) in general spine surgery. However, there are limited data of its use in oncological spine patients.
Methods: A systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed, Cochrane, OVID, and Embase databases were searched. Search terms: “tranexamic acid”, “aprotinin,” “aminocaproic acid,” “spine surgery,” “spine tumors,” and “spine oncology.” Included studies were full text publications written in English with patients treated with either agent or who had surgery for oncological spine disease (OSD).
Results: Seven hundred results were reviewed form the different databases, seven were selected. A total of 408 patients underwent spine surgery for OSD and received antifibrinolytics. There was a male predominance (55.2%) and mean age ranged from 43 to 62 years. The most common tumor operated was metastatic renal cancer, followed by breast and lung. Most studies administered TXA as a bolus followed by an infusion during surgery. Median blood loss was of 667 mL (253.3–1480 mL). Patients with TXA required 1–2 units less of transfusion and had 56–63 mL less of postoperative drainage versus no TXA. The median incidence of deep venous thrombosis (DVT) was 2.95% (0–7.9%) and for pulmonary embolism (PE) was 4.25% (0–14.3%). The use of TXA reduced intraoperative blood loss, transfusions and reduced postoperative surgical drainage output compared to no TXA use in patients with OSD.
Conclusion: In this review, we found that TXA may diminish intraoperative blood loss, the need for transfusion and postoperative drainage from surgical drains when used in OSD without major increase in rates of DVT or PE.
Keywords: Antifibrinolytics, Metastatic spine disease, Spine oncology, Spine surgery, Tranexamic acid
INTRODUCTION
Metastases are indeed the most common type of tumor in the spine.[
Patients with cancer present commonly with nutritional deficiencies and sarcopenia compared to those with spine deformity or degenerative disorders that need spine surgery.[
Given the exponential growth of spine surgery, in particular larger surgeries, recent studies have shown the benefits of using antifibrinolytic therapy in spine surgery to limit blood loss. A recent meta-analysis by Li et al.[
In this study, we aim to investigate the use of antifibrinolytic therapy in patients undergoing spine surgery for tumors (metastatic or primary) of the spine with particular interest of blood loss parameters and complications.
MATERIALS AND METHODS
A systematic review of the literature was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[
Search strategy and screening
Databases were used included: PubMed, PubMed Central, Cochrane Library, Clinicaltrials.gov, and Embase. The search was catered to gather English language articles published from any beginning date to September 2021. The following antifibrinolytics agents were included: tranexamic acid (TXA), Aminocaproic Acid and Aprotinin. Combinations and variations of key phrases including: “tranexamic acid,” “aprotinin,” “aminocaproic acid,” “Spine tumors,” “spine oncology,” “spine metastasis,” “spine surgery,” “spine fusion,” “Spine fixation,” “spine decompression,” “spinal surgery” with the use of Boolean AND and OR in multiple configurations.
Inclusion and exclusion criteria
Eligible studies used antifibrinolytics during their spinal surgery for spine oncological diseases (metastatic or primary). The inclusion criteria for the reviewed articles included case reports, case series, retrospective, and prospective studies as well. Studies were limited to those written in the English language. Screened studies such as abstracts, posters, indexes, commentaries, author notes, and literature reviews were excluded from the study.
Data extraction
All data were taken directly from tables, figures, and texts of included articles. The relevant data was extracted and placed into a custom table which included article’s first author and year published, study type, number of patients included, type of antifibrinolytics agent, age and sex details, oncological diagnosis, treatment approach, outcome, and venous thromboembolism events. When data were unclear or unspecified, it was noted in the table as “-”.
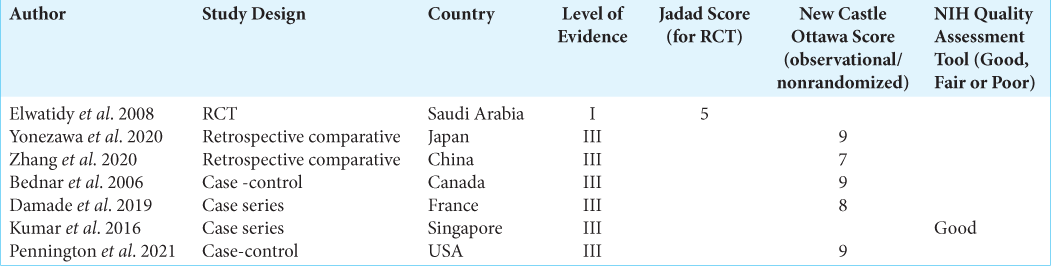
Characteristics and quality of included studies
To assess the risk of bias of randomized controlled trials, the Jadad Scale[
Statistical analysis
Although initially planned, a meta-analysis was not performed given the heterogeneity of data including multiple study design, poor quality of reports, missing data, different antifibrinolytics administration protocols, and lack of control group in most studies as well as the inconsistency in reporting clinical outcomes. Therefore, only descriptive statistics were performed. Data were presented as mean and standard deviation or median and interquartile range, if appropriate. For categorical variables, absolute values and percentages were used.
RESULTS
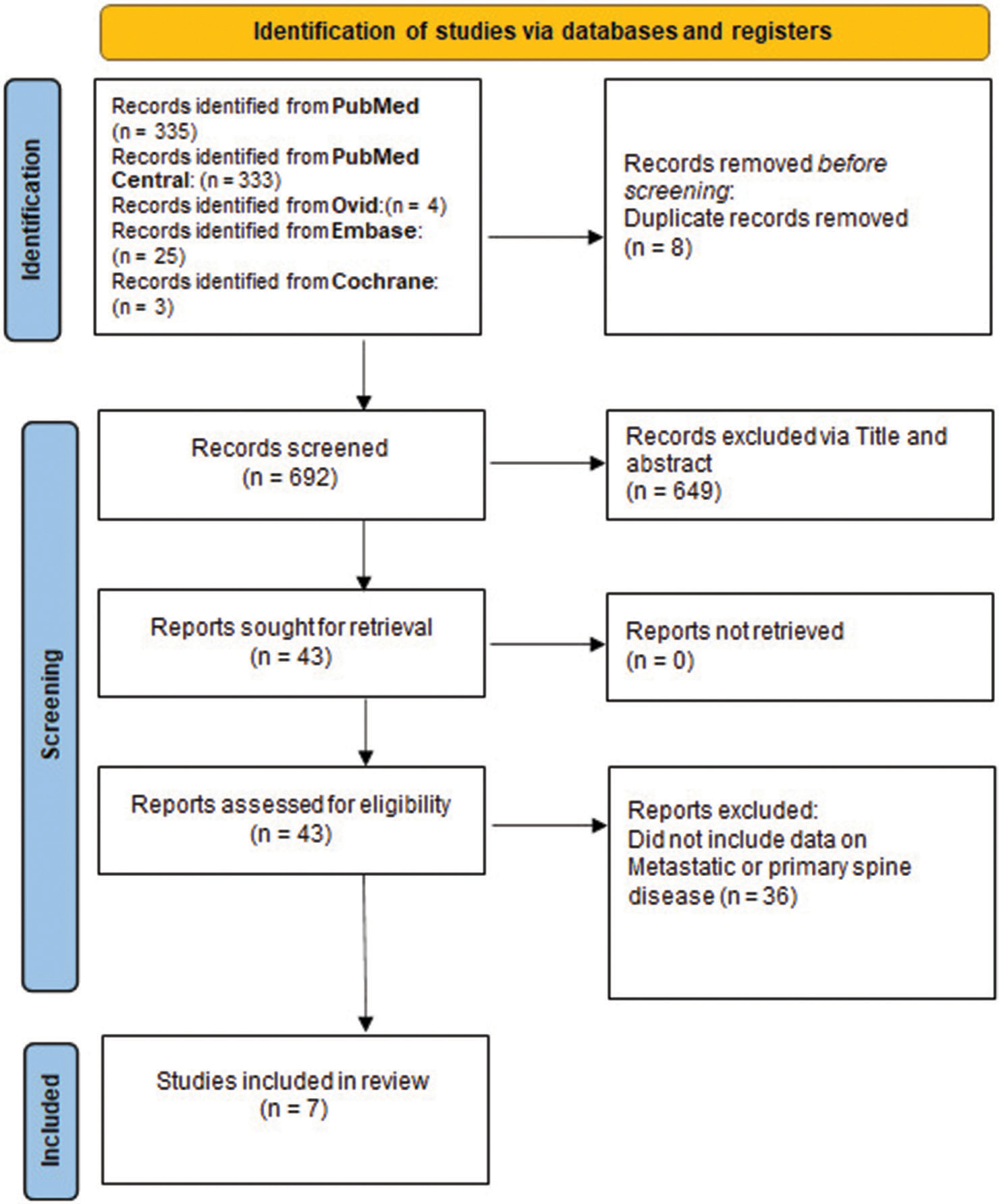
A total of 700 articles were found in the different databases. After selection by title and abstract, 43 articles were downloaded for full text review. Of these, 36 articles were removed given lack of data for spine oncology patients and seven articles[
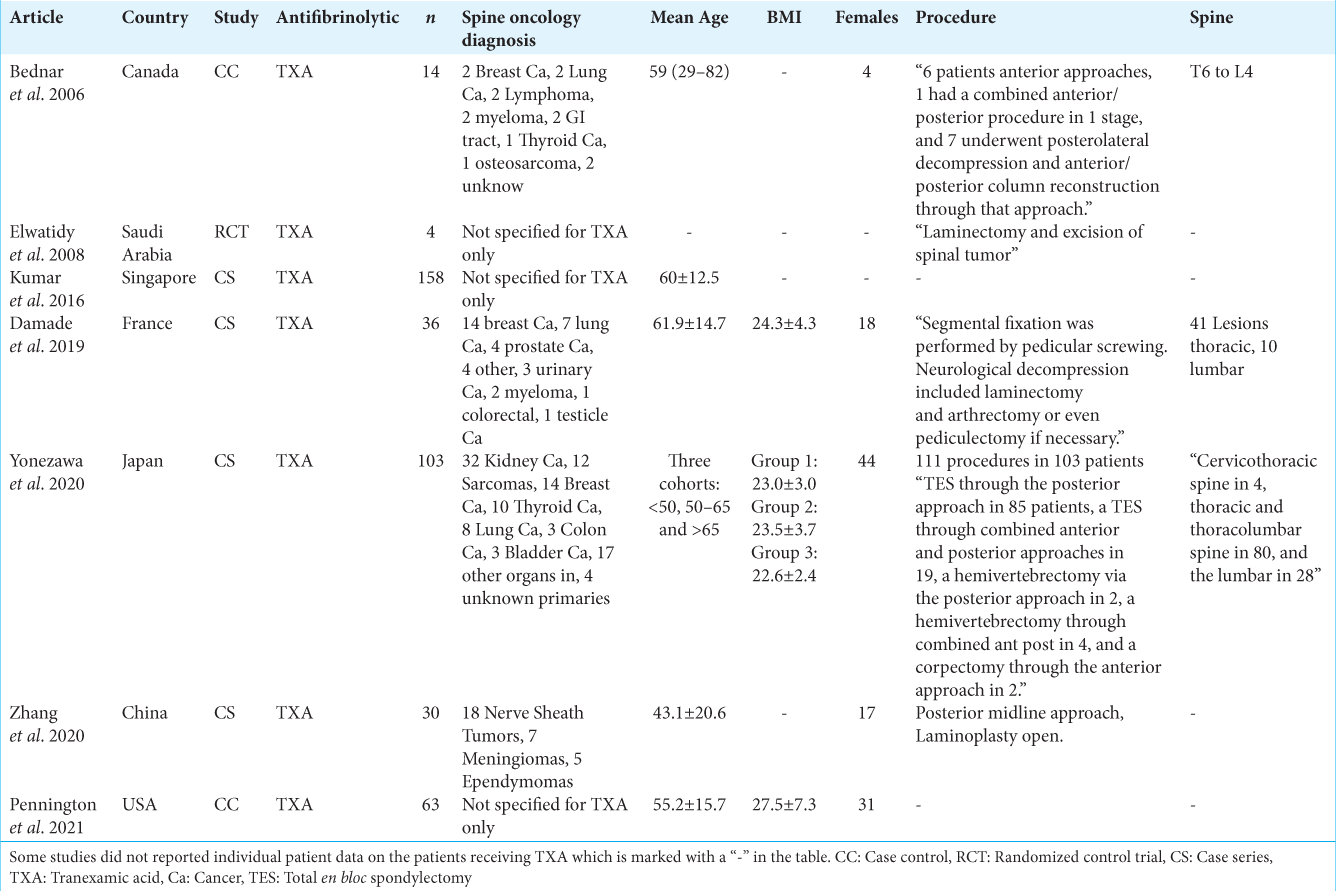
All studies used TXA as the antifibrinolytic of choice.
The studies reported that the use of TXA was done due to the high risk of intraoperative bleeding in these patients. All the studies reported that their patients’ cohort who received TXA did not have coagulopathy before surgery measured by preoperatively blood coagulation analysis. Moreover, four studies[
TXA dosing and timing
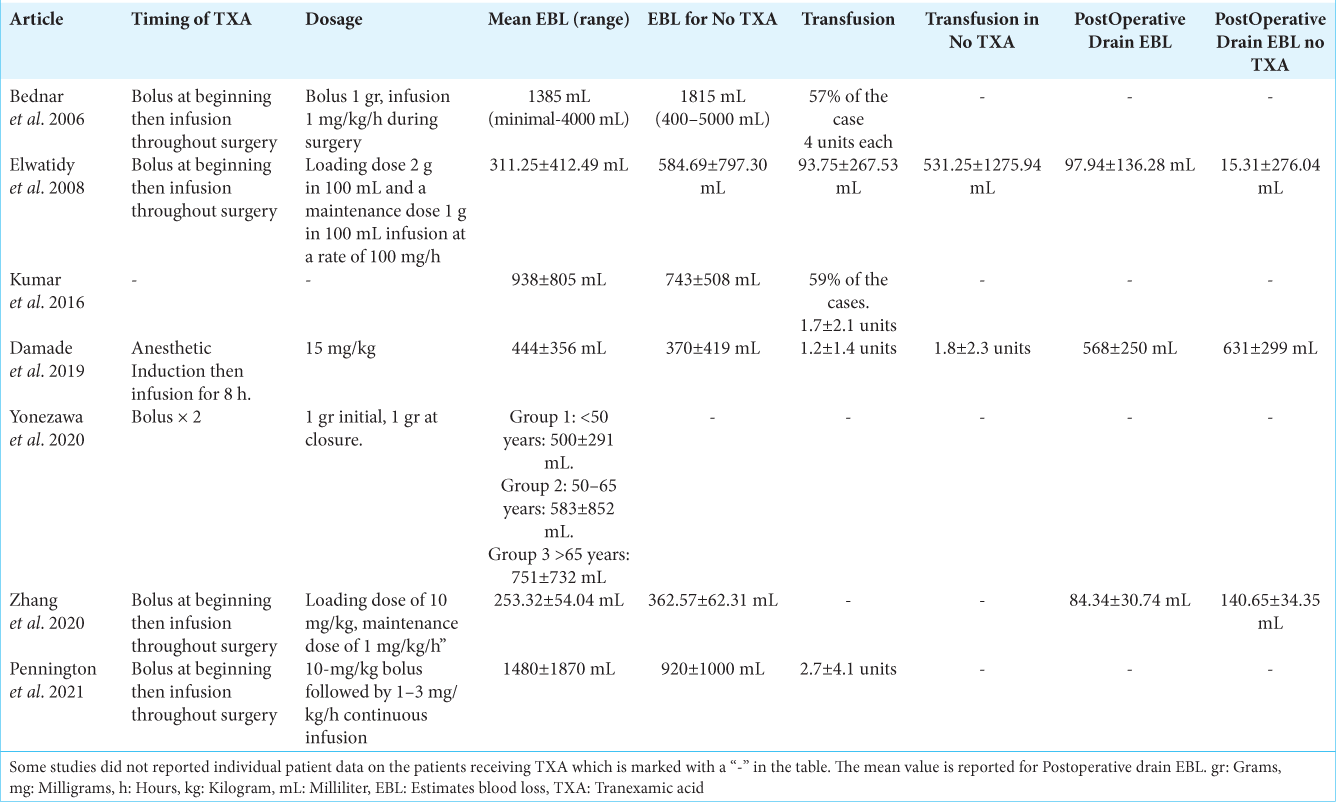
Six out of the seven studies reported the dosage used. The TXA dose range was from 1 mg/kg to up to 20 mg/kg with 10 mg/kg being the most common. There were two methods for the timing of TXA administration within the different studies: (a) one dose bolus at the beginning and (b) bolus plus a maintenance infusion during surgery. Five out of the six studies that reported the dose performed a maintenance infusion of TXA throughout the surgery. One study[
Intraoperative blood loss
All studies reported intraoperative blood loss [
Transfusion
Five studies reported the need and amount of blood transfusion [
Postoperative blood loss
For this section, we compiled studies that reported postoperative blood loss in surgical drains. Three studies reporting the use of postoperative drains. The study by Zhang et al.[
Deep venous thrombosis (DVT)
Six out of the seven studies reported the incidence of DVT. Median combined DVT for the studies was 2.95%. In individual studies, fours studies reported 0 DVT events and the highest reported was by Pennington et al.[
Pulmonary embolism (PE)
Six out of the seven studies reported the incidence of PE. Four studies reported an incidence of 0% while using TXA. The higher incidence of PE was reported by Pennington et al.[
DISCUSSION
Given the baseline frailty of cancer patients, adjuvants for intraoperative hemorrhage control while undergoing spine surgery are needed to avoid massive blood loss that may complicate their postoperative course. In addition, cancer patients tend to be anemic at baseline before surgery[
In this study, we found that patients who received TXA undergoing treatment for tumors in the spine (metastatic and primary) had diminished blood loss, diminished postoperative blood loss (from surgical drains), and less need for transfusions without major increase in venous thromboembolism events. Nonetheless, some individual studies showed no statistically significant difference in blood loss between TXA and non-TXA groups.
Antifibrinolytics agents have shown good results in spine deformity surgery, but they are less studied in spine oncology. Tsantes et al.[
On a broader perspective, the pooled median EBL for the included studies with TXA was 667 mL (range 252–1480 mL) when compared to surgeries such as minimally invasive tumor resection,[
Although only seven studies were selected these included an overall sample of 408 patients, this is quite comparable with a meta-analysis by Li et al.[
Interestingly, our results showed a wide variety of spinal oncological diseases. Metastatic cancer was the most common diagnosis with renal carcinoma as the number one followed by breast cancer. Additional tumors included sarcomas, thyroid metastases, lung metastases, nerve sheath tumors, myeloma, and meningiomas which show the benefit of TXA in a variety of oncological spine disease treated surgically making our study quite unique in terms of the diversity of diagnosis. To the best of our knowledge, this is the first study to incorporate different oncological diagnosis and the use of TXA during spine surgery.
Only two studies reported preoperative embolization; Yonezawa et al. who presented the largest series of renal carcinoma metastasis performed embolization in all their patients[
There was variety in the dosing and method of administering TXA in the different studies; the majority of the studies performed an initial bolus of TXA and continued an infusion throughout the surgery. As with the published literature for spine deformity, there is no clear agreement on the optimal dosing. A recent review and meta-analysis favored a lower dose of TXA (200–500 mg) compared to higher dose (1–3 g).[
Our results showed that despite the administration of TXA, spine tumor patients do not appear to be at an unusual increased risk of venous thromboembolism events, one of the major concerns of using these hemostatic agents. Several of the included studies reported 0% incidence of DVT/PE.[
There is a lack of randomized trials in spine oncology and use of antifibrinolytics agents. Despite our extensive database search only one RCT was found, and this study included a mix of degenerative and oncological patients, the rest of the studies were retrospective case series from individual hospitals. Ideally, a multi-center prospective randomized trial may help validate our findings. In particular, if there are any differences in terms of the spinal oncological diagnosis and the effectiveness of the antifibrinolytics agent. Our results suggest that there is probably no difference.
With the advancement of separation surgery[
Limitations
The study has several limitations. The majority of included studies are retrospective case series which renders our results of a low evidence given the available studies. The highest level of evidence in this series is a Level I for Elwaitidy et al.[
CONCLUSION
The use of antifibrinolytics agents in surgery for spine tumors is not well studied. In this systematic review, we found that the use of TXA during surgery for spine tumors may diminish intraoperative blood loss, the need for transfusion and postoperative drainage from surgical drains without major increase in rates of DVT or PE. Additional randomized controlled multi-center studies are needed to further support these findings.
Author’s Declaration
Portions of this work were presented as an Oral presentation at the 2022 AANS/CNS Spine summit in Las Vegas, NV.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016. 8: e519
2. Bednar DA, Bednar VA, Chaudhary A, Farrokhyar F, Farroukhyar F. Tranexamic acid for hemostasis in the surgical treatment of metastatic tumors of the spine. Spine (Phila Pa 1976). 2006. 31: 954-7
3. Bozzetti F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017. 28: 2107-18
4. Damade C, Tesson G, Gilard V, Vigny S, Foulongne E, Gauthé R. Blood loss and perioperative transfusions related to surgery for spinal tumors. Relevance of tranexamic acid. Neurochirurgie. 2019. 65: 377-81
5. Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: A prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008. 33: 2577-80
6. Fatima N, Barra ME, Roberts RJ, Massaad E, Hadzipasic M, Shankar GM. Advances in surgical hemostasis: A comprehensive review and meta-analysis on topical tranexamic acid in spinal deformity surgery. Neurosurg Rev. 2021. 44: 163-75
7. Godersky JC, Smoker WR, Knutzon R. Use of magnetic resonance imaging in the evaluation of metastatic spinal disease. Neurosurgery. 1987. 21: 676-80
8. Hockley A, Ge D, Vasquez-Montes D, Moawad MA, Passias PG, Errico TJ. Minimally invasive versus open transforaminal lumbar interbody fusion surgery: An analysis of opioids, nonopioid analgesics, and perioperative characteristics. Glob Spine J. 2019. 9: 624-9
9. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clin Trials. 1996. 17: 1-12
10. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007. 110: 2339-46
11. Kumar N, Zaw AS, Khine HE, Maharajan K, Wai KL, Tan B. Blood loss and transfusion requirements in metastatic spinal tumor surgery: Evaluation of influencing factors. Ann Surg Oncol. 2016. 23: 2079-86
12. Li G, Sun TW, Luo G, Zhang C. Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: A meta-analysis. Eur Spine J. 2017. 26: 140-54
13. Li ZJ, Fu X, Xing D, Zhang HF, Zang JC, Ma XL. Is tranexamic acid effective and safe in spinal surgery? A meta-analysis of randomized controlled trials. Eur Spine J. 2013. 22: 1950-7
14. Lu VM, Kerezoudis P, Gilder HE, McCutcheon BA, Phan K, Bydon M. Minimally invasive surgery versus open surgery spinal fusion for spondylolisthesis: A systematic review and meta-analysis. Spine. 2017. 42: E177-85
15. Moussazadeh N, Laufer I, Yamada Y, Bilsky MH. Separation surgery for spinal metastases: Effect of spinal radiosurgery on surgical treatment goals. Cancer Control. 2014. 21: 168-74
16. National Heart L and Blood Institute, National Institutes of Health, US Department of Health and Human Services. Available from: https://www.nhlbinih.gov/health-topics/study-quality-assessmen-tools [Last accessed on 2022 Sep 11].
17. Orenday-Barraza JM, Cavagnaro MJ, Avila MJ, Strouse IM, Dowell A, Kisana H. 10-year trends in the surgical management of patients with spinal metastases: A scoping review. World Neurosurg. 2021. 157: 170-86
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
19. Pennington Z, Ehresman J, Schilling A, Feghali J, Hersh AM, Hung B. Influence of tranexamic acid use on venous thromboembolism risk in patients undergoing surgery for spine tumors. J Neurosurg Spine. 2021. 35: 663-73
20. Pritchyk KM, Schiff BA, Newkirk KA, Krowiak E, Deeb ZE. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002. 112: 1598-602
21. Raman T, Varlotta C, Vasquez-Montes D, Buckland AJ, Errico TJ. The use of tranexamic acid in adult spinal deformity: Is there an optimal dosing strategy?. Spine J. 2019. 19: 1690-7
22. Rothrock RJ, Barzilai O, Reiner AS, Lis E, Schmitt AM, Higginson DS. Survival trends after surgery for spinal metastatic tumors: 20-Year cancer center experience. Neurosurgery. 2021. 88: 402-12
23. Bozzetti F, Mariani L, Lo Vullo S, Amerio ML, Biffi R. The nutritional risk in oncology: A study of 1,453 cancer outpatients. Support Care Cancer. 2012. 20: 1919-28
24. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013. 122: 1712-23
25. Tsantes AG, Trikoupis IG, Papadopoulos DV, Goumenos S, Piovani D, Nikolopoulos GK. The safety and efficacy of tranexamic acid in oncology patients undergoing endoprosthetic reconstruction and a ROTEM-based evaluation of their hemostatic profile: A pilot study. Cancers (Basel). 2021. 13: 3951
26. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, editors. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Oxford: University of Ottawa; 2000. p.
27. Wright E, Ricciardi F, Arts M, Buchowski JM, Chung CK, Coppes M. Metastatic spine tumor epidemiology: Comparison of trends in surgery across two decades and three continents. World Neurosurg. 2018. 114: e809-17
28. Yonezawa N, Murakami H, Demura S, Kato S, Yoshioka K, Shinmura K. Perioperative complications and prognosis of curative surgical resection for spinal metastases in elderly patients. World Neurosurg. 2020. 137: e144-51
29. Zairi F, Arikat A, Allaoui M, Marinho P, Assaker R. Minimally invasive decompression and stabilization for the management of thoracolumbar spine metastasis. J Neurosurg Spine. 2012. 17: 19-23
30. Zhang HZ, Dong L, Wang HM, Hu F, Shao Q, Chen X. Safety and efficacy of tranexamic acid in spinal canal tumors: A retrospective cohort study. Br J Neurosurg. 2020. 34: 313-5