- College of Medicine, King Saud University, Riyadh, Saudi Arabia
- Department of Surgery, Division of Neurosurgery, King Saud University Medical City, Riyadh, Saudi Arabia
- Department of Surgery, Division of Neurosurgery, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
Correspondence Address:
Alwaleed Abdulrahman Alsaleh, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_1011_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulaziz Alomayri1, Ali A. Basalamah2, Alwaleed Abdulrahman Alsaleh1, Sultan Alreshood2, Abdulrahman Aldakkan3. Aortoiliac occlusion mimicking cauda equina syndrome, a diagnostic dilemma: A case report and review of the literature. 29-Mar-2024;15:107
How to cite this URL: Abdulaziz Alomayri1, Ali A. Basalamah2, Alwaleed Abdulrahman Alsaleh1, Sultan Alreshood2, Abdulrahman Aldakkan3. Aortoiliac occlusion mimicking cauda equina syndrome, a diagnostic dilemma: A case report and review of the literature. 29-Mar-2024;15:107. Available from: https://surgicalneurologyint.com/surgicalint-articles/12832/
Abstract
Background: Cauda equina syndrome (CES) is a consequence of a variety of etiologies. CES is most commonly due to compression of the thecal sac and nerve roots by a massive disc herniation. However, it rarely presents secondary to aortic occlusion. Aortoiliac occlusive disorder is usually associated with chronic claudication, erectile dysfunction, and diminished lower limb pulses. Acute aortic occlusion, however, is associated with serious complications such as spinal cord infarction and ischemia. It is also associated with a high risk of morbidity and mortality. Moreover, it poses a diagnostic challenge and may be overlooked. This report emphasizes the importance of considering vascular etiology as a differential diagnosis for CES.
Case Description: This case report describes a unique case of aortic occlusion mimicking CES in a 56-year-old female patient.
Conclusion: For patients presenting with cauda equina symptomatology, it is critical to consider vascular etiology, especially for those with cardiovascular risk factors. Spine surgeons and emergency physicians should maintain a high index of suspicion for vascular etiologies and consider appropriate imaging studies to promote early diagnosis and intervention to prevent subsequent neurological and life-threatening consequences.
Keywords: Abdominal aorta, Acute aortoiliac occlusion, Cauda equina syndrome, Emergency physician, Spine surgeon
INTRODUCTION
Cauda equina syndrome (CES) is an uncommon condition that affects <1 in 100,000 patients annually. This syndrome is a consequence of a variety of etiologies, most commonly compression of the thecal sac and nerve roots by massive disc herniation.[
This report emphasizes the importance of considering vascular etiology as a differential diagnosis for CES. Here, we present a challenging clinical scenario of a unique case of aortic occlusion mimicking CES.
CASE PRESENTATION
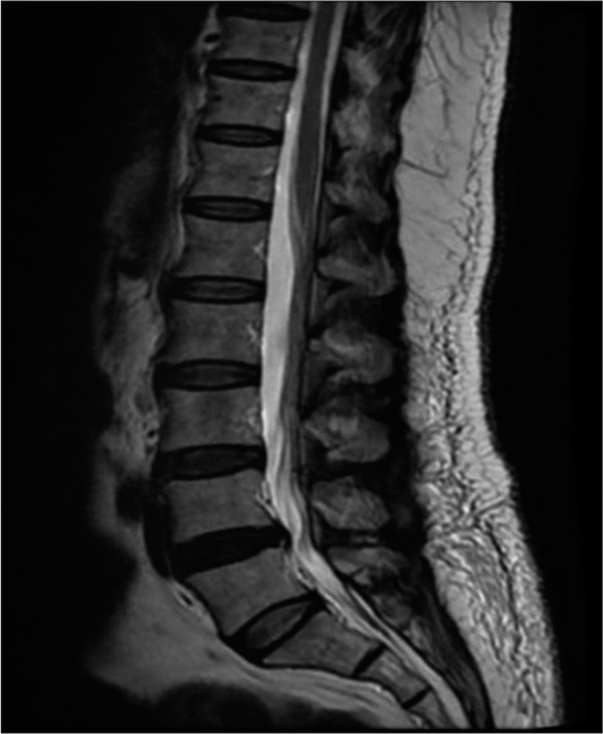
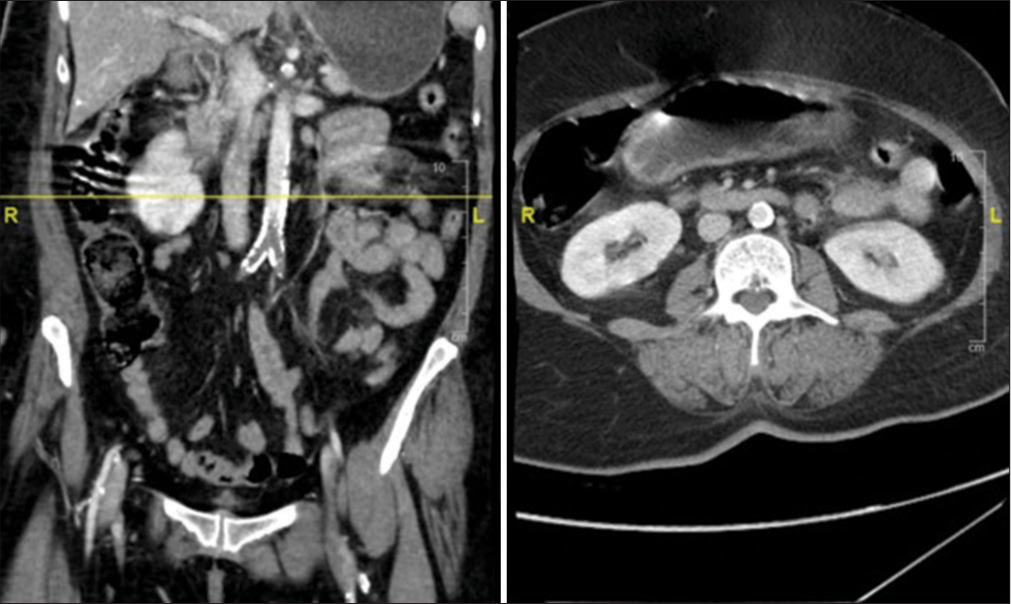
A 56-year-old female patient with hypertension, hyperlipidemia, and ischemic heart disease underwent coronary artery bypass grafting in 2016. She had a recent history of non-ST-segment elevation myocardial infarction and dual antiplatelet therapy. She was brought to the emergency department with the chief complaint of acute onset of severe lower back pain, bilateral lower limb weakness, non-dermatomal bilateral lower limb pain, and urinary incontinence. Upon examination, the patient experienced distress and pain despite receiving appropriate analgesic medications, including narcotics. The patient was conscious, febrile, and hypertensive (220/104). The power in the left lower limb was 0/5, and 3/5 in the right lower limb in all muscle groups. She had absent deep tendon reflexes and diminished sensation in both lower limbs. The anal tone was weak, but no saddle anesthesia was present. The lower limbs were not cold, and pedal pulses were palpable but diminished. An emergent magnetic resonance imaging (MRI) of the lumbar–sacral spine was performed and showed an L4– L5 diffuse disk bulge indenting the ventral thecal sac and causing bilateral mild-to-moderate neural foraminal stenosis, mild spinal canal stenosis, and bilateral facet arthrosis [
The patient then underwent emergent bilateral aortoiliac embolectomy and bilateral four-compartment lower limb fasciotomy. Postoperatively, there was no improvement in neurological function. She was started on heparin infusion, and dual antiplatelet therapy was initiated. The patient’s condition continued to deteriorate a few days later, and she died due to a cardiac event.
DISCUSSION
Acute aortic occlusion (AAO) is an uncommon vascular disorder and emergency that is associated with a mortality rate ranging from 20% to 75%.[
Chronic aortoiliac occlusive disorder is likely to manifest as chronic erectile disorder, claudication, diminished lower limb pulses, cool extremities, cyanotic extremities, compartment syndrome, and myonecrosis.[
Certain aspects of management, including distinguishing between embolism and thrombosis etiologies before surgery, the use of aortography, the best initial treatment modality, the preferred course of management, and the role of long-term or permanent anticoagulation, are subjects of debate.[
Revascularization/reperfusion interventions may include thrombectomy, embolectomy, thrombolysis, extra-anatomic bypass, and direct repair.[
Patients with AAO may present with clinical pictures mimicking CES, which may lead to a diagnostic puzzle. To preclude misdiagnosis, emergency medicine physicians and spine surgeons must have a high index of suspicion regarding vascular etiology. Along with a detailed neurological examination, peripheral vascular examination should not be overlooked. The consideration of vascular etiologies should be even higher, especially if a spinal MRI does not suggest a compressive neurological etiology and/or a spinal cord lesion.
CONCLUSION
For patients presenting with cauda equina symptomatology, it is critical to consider vascular etiology, especially for those with cardiovascular risk factors. Spine surgeons and emergency physicians should maintain a high index of suspicion for vascular etiologies and consider appropriate imaging studies to promote early diagnosis and intervention to prevent subsequent neurological and life-threatening consequences.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
American manuscript editors.
References
1. Ahn UM, Ahn NU, Buchowski JM, Garrett ES, Sieber AN, Kostuik JP. Cauda equina syndrome secondary to lumbar disc herniation: A meta-analysis of surgical outcomes. Spine. 2000. 25: 1515-22
2. Azzarone M, De Troia A, Iazzolino L, Nabulsi B, Tecchio T. Hybrid treatment of acute abdominal aortic thrombosis presenting with paraplegia. Ann Vasc Surg. 2016. 33: 228.e5-8
3. Babu SC, Shah PM, Nitahara J. Acute aortic occlusion--factors that influence outcome. J Vasc Surg. 1995. 21: 567-75 discussion 573-5
4. Bakas JM, Bijdevaate DC, Lauw MN, van Veelen-Vincent ML, van Rijn MJ. A case of complete resolution of cauda equina syndrome caused by extensive iliocaval thrombosis: The role of thrombolysis and venous stents. J Endovasc Ther. 2023. p. 15266028231179596
5. Barraclough K. Cauda equina syndrome. BMJ. 2021. 372: n32
6. Bolduc M, Clayson S, Madras P. Acute aortic thrombosis presenting as painless paraplegia. J Cardiovasc Surg (Torino). 1989. 30: 506-8
7. Clair DG, Beach JM. Strategies for managing aortoiliac occlusions: Access, treatment and outcomes. Expert Rev Cardiovasc Ther. 2015. 13: 551-63
8. Dossa CD, Shepard AD, Reddy DJ, Jones CM, Elliott JP, Smith RF. Acute aortic occlusion. A 40-year experience. Arch Surg. 1994. 129: 603-7 discussion 607-8
9. Fraser S, Roberts L, Murphy E. Cauda equina syndrome: A literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009. 90: 1964-8
10. Grip O, Wanhainen A, Björck M. Temporal trends and management of acute aortic occlusion: A 21 yearexperience. Eur J Vasc Endovasc Surg. 2019. 58: 690-6
11. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW, Bolen MA. 2022 ACC/AHA Guideline for the diagnosis and management of aortic disease: A report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. 2022. 146: e334-482
12. Kalayjian R, Herzig RH, Cohen AM, Hutton MC. Thrombosis of the aorta is caused by mucormycosis. South Med J. 1988. 81: 1180-2
13. Kapetanakis S, Chaniotakis C, Kazakos C, Papathanasiou JV. Cauda equina syndrome due to lumbar disc herniation: A review of the literature. Folia Med (Plovdiv). 2017. 59: 377-86
14. Long B, Koyfman A, Gottlieb M. Evaluation and management of cauda equina syndrome in the emergency department. Am J Emerg Med. 2020. 38: 143-8
15. Nishikawa H, Miyakoshi S, Nishimura S, Seki A, Honda K. A case of aortic intimal sarcoma manifested with acutely occurring hypertension and aortic occlusion. Heart Vessels. 1989. 5: 54-8
16. Over DR, Deaver J, Pumphery CY. Acute aortic occlusion with spinal cord infarction. Fed Pract. 2018. 35: 32-5
17. Paone R, Romsi P. A case of acute aortoiliac occlusive disease presenting as cauda equina syndrome and Fournier´s gangrene. Case Rep Surg. 2019. 2019: 4027460
18. Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989. 68: 282-92
19. Wong SS, Roche-Nagle G, Oreopoulos G. Acute thrombosis of an abdominal aortic aneurysm presenting as cauda equina syndrome. J Vasc Surg. 2013. 57: 218-20
20. Yamamoto H, Yamamoto F, Tanaka F, Motokawa M, Shiroto K, Yamaura G. Acute occlusion of the abdominal aorta with concomitant internal iliac artery occlusion. Ann Thorac Cardiovasc Surg. 2011. 17: 422-7