- Department of Neurosurgery, SUNY Upstate Medical University, Syracuse, New York, United States
Correspondence Address:
Emily Harland, Department of Neurosurgery, SUNY Upstate Medical University, Syracuse, New York, United States.
DOI:10.25259/SNI_577_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Emily Harland, Justin Oh, Matthew Protas, Satish Krishnamurthy. Are variations in ventricular catheter placement related to design of the catheter? A single-center cohort study. 25-Oct-2024;15:385
How to cite this URL: Emily Harland, Justin Oh, Matthew Protas, Satish Krishnamurthy. Are variations in ventricular catheter placement related to design of the catheter? A single-center cohort study. 25-Oct-2024;15:385. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13162
Abstract
Background:The use of intracranial catheters is a common procedure used for neurosurgical patients with a variety of pathologies. Despite its frequency of use, shunt failure and revision have been reported to be a common problem. Given that depth of insertion can significantly affect the catheter tip position, a single institution retrospective chart review was performed to examine the accuracy of shunt and external ventricular drain (EVD) placement.
Methods:Computed tomography (CT) images of the head following shunt or ventriculostomy insertion were analyzed to determine the delta between the final length of the intracranial catheter and the intended depth described in the operative notes.
Results:We found that there was a statistically significant difference in the accuracy of placement when comparing EVDs to shunts. The most used EVDs at our institution are marked with a solid black line in increments spaced 2 cm apart. The most used ventricular shunt catheter has a marking at 5 cm and 10 cm from the tip of the catheter. We believe that the visual confirmation that is afforded by metric unit markings on the EVD allows for better final placement of the catheter at the outer table of the calvarium.
Conclusion:The addition of regular millimeter metric unit markings by the manufacturer is imperative in decreasing the chances of error in the insertion of ventricular catheters and preventing potential neurovascular injury to the surrounding structures.
Keywords: Cerebrospinal fluid, External ventricular drain, Neurosurgical technique
INTRODUCTION
Intracranial catheters such as shunts and external ventricular drains (EVDs) are the most common procedures in neurosurgical patients that allow for cerebrospinal fluid (CSF) diversion or monitoring of intracranial pressures. This treatment option is used for a variety of pathologies, such as severe traumatic brain injury to hydrocephalus of various etiologies.[
One of the major reasons for shunt revision is obstruction of the proximal catheter related to the placement of the intracranial catheter.[
According to a contemporary literature review conducted by Vlasak et al., the average cost of diagnosing, material, and procedural costs of placing ventricular catheters was estimated to be between $1300 and $3200/patient.[
Intraventricular catheter insertion is most commonly done with a freehand technique utilizing cranial landmarks, and depending on the type of shunt system being used, the proximal catheter must be cut to size and connected to the shunt valve. During this maneuver, the proximal catheter depth may be altered, and the absence of metric unit markings leaves few options for confirming the depth of the catheter during the process of connecting the catheter to the valve. There are a variety of proximal shunt catheters that are Food and Drug Administration approved and marketed without continuous markings on the catheter to determine the depth of insertion, such as centimeters or millimeters with numbers. While these markings are common in other high-risk devices, such as an endotracheal tube, the ventricular catheters have markings at each centimeter without any numbers that would help the clinician determine the depth immediately. At our institution, we use the Codman Bactiseal EVD catheter that has numerical metric unit markings in two cm intervals. This design forces the person performing the procedure to guess the depth of the catheter between the two markings. Many neurosurgeons use other methods to mark the depth of the catheter desired in a particular patient with either a suture tie or a pen mark. Both methods are fraught with error as the tie can slip, and a pen mark can be erased with blood or fluid. Most of the shunt catheters have only markings every 5 cm, and a dot and no numbers represent these. Given that depth of insertion can significantly affect the catheter tip position, we conducted a single institution retrospective chart review to examine the accuracy of shunt and EVD placement. We analyzed computed tomography (CT) images of the head following shunt or ventriculostomy insertion to analyze the delta between the final length of the intracranial catheter and the intended depth described in the operative notes. We hypothesized that EVD catheters are placed more accurately than shunt catheters due to the visual ability to confirm the depth of insertion with EVD catheters.
MATERIALS AND METHODS
We performed a retrospective chart review at a single institution and queried shunt insertions, and EVD insertions performed between 2018 and 2022 in subjects over 18 years old. This study was approved by the State University of New York Upstate Medical University institutional review board as an exempt study. Demographic data, indication for operation, need for shunt revision or EVD replacement, use of navigation, and CT head images were collected. Patient consent was not obtained as we only performed a retrospective review of operative scans. We chose only to evaluate frontal approach catheter placements to be able to compare EVD insertions with shunt insertions.
3D slicer, a free, open-source platform, was used to perform measurements of the intracranial catheter length from the position at the outer table of the calvarium to the catheter tip. We preferentially used axial CT head bone window scans, which provided the most accurate visual of the catheter’s pathway. The software allowed us to follow the trajectory of the catheters slice-by-slice through the CT scans allowing for the most accurate measurements possible. Catheter measurements were performed to the second decimal place. Medical records were reviewed for each subject, and the intended depth of the intracranial catheter placement was collected.
A paired t-test was used to evaluate the mean difference between the measured catheter lengths and descriptive catheter lengths, which we termed delta. Analysis of variance was used to compare variance in described catheter lengths stratified by indication for the procedure. The significance level was set to P < 0.05.
RESULTS
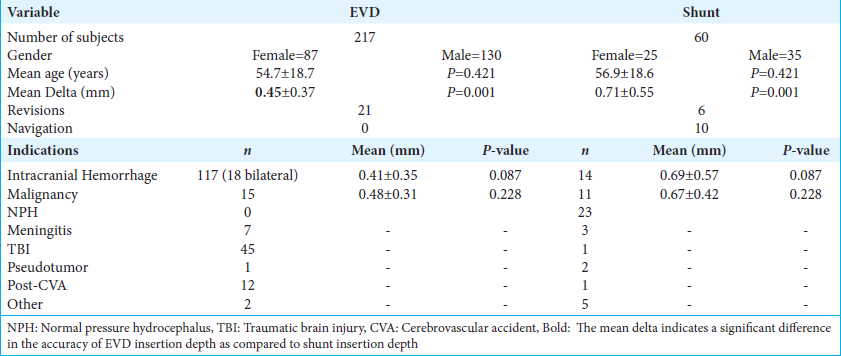
There were a total of 217 EVD insertions and 60 ventricular shunt insertions performed at our institution between 2018 and 2022 after excluding subjects that underwent parietal/occipital catheter insertions as well as subjects that did not have an intended catheter insertion depth in their operative notes. The mean age of patients in the shunt group was 54.7 years, and 56.9 years in the shunt group. The indications for EVD insertion were intracranial hemorrhage (n = 135), hydrocephalus due to leptomeningeal neoplastic process or obstructive neoplastic process (n = 15), central nervous system infection (n = 7), traumatic brain injury (n = 41), fulminant pseudotumor cerebri (n = 1), ischemic stroke (n = 12), and other indications (n = 2). The indications for shunt insertions were posthemorrhagic hydrocephalus (n = 14), postinfectious hydrocephalus (n = 3), hydrocephalus due to neoplastic processes (n = 11), posttraumatic hydrocephalus (n = 1), normal pressure hydrocephalus (n = 23), pseudotumor cerebri (n = 2), poststroke hydrocephalus (n = 1), and other indications (n = 5).
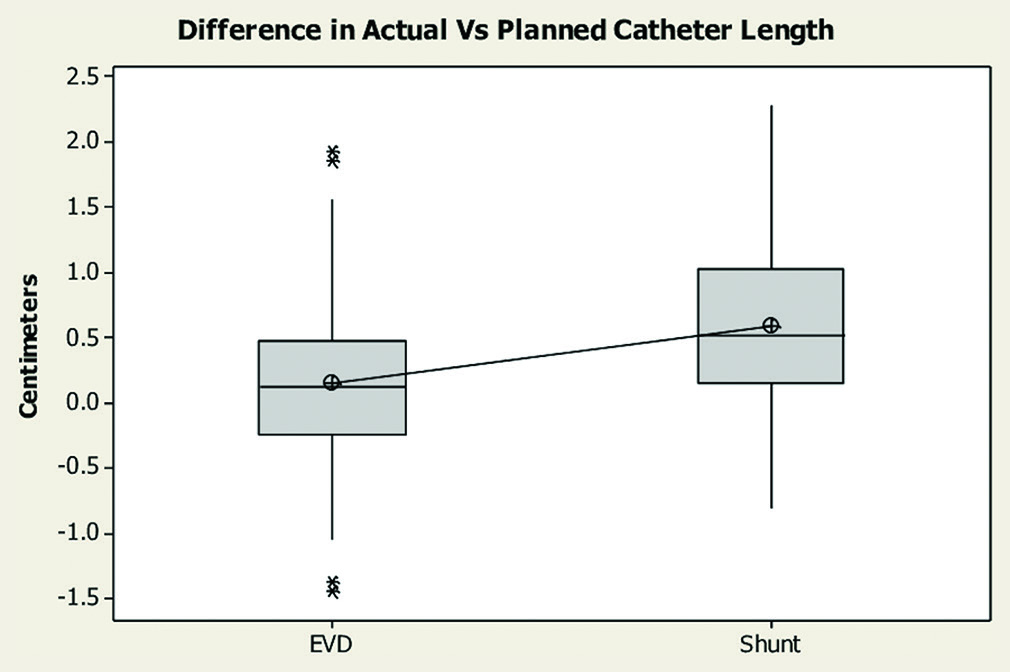
The mean delta, or the difference between the measured catheter length on the postoperative scan and the intended depth in the operative note, in the EVD group was 0.45 mm (standard deviation [SD] 0.37 mm), and in the shunt group was 0.71 mm (SD 0.55 mm). There was a statistically significant difference between the measured depth and intended depth in the shunt group when compared to the EVD group (P < 0.001) 95% confidence interval [−0.407, −0.109]. This indicates that shunt catheters, which have less frequent markings as compared to EVDs, were inserted less precisely [
Figure 1:
Box plot demonstrating differences between measured catheter length on the postoperative scan and the intended depth in the operative note between the external ventricular drain (EVD) and the shunt groups. EVD group mean delta 0.45 mm (standard deviation [SD] 0.37 mm). Shunt group mean delta 0.71 mm (SD 0.55 mm). P < 0.001, 95% confidence interval [−0.407, −0.109]. Asterisks represent significant outliers.
DISCUSSION
Use error is quite common in the practice of medicine, as underscored by the 1999 Institute of Medicine report, To Err Is Human.[
Key results
We found that there was a statistically significant difference between the accuracy of insertion depth when comparing EVD insertions to shunt insertions. The standard EVD catheter that is used at our institution is the Codman Bactiseal catheter (Integra LifeSciences) and these are marked with a solid black line in increments spaced 2 cm apart. The most commonly used ventricular shunt catheter used at our institution is the Codman MEDOS ventricular catheter (Integra LifeSciences), which has a marking at 5 cm and 10 cm from the tip of the catheter. We believe that the visual confirmation that is afforded by metric unit markings on the EVD allows for better final placement of the catheter at the outer table of the calvarium. At our institution, EVD catheters are secured at the exit site before closing the incision, ensuring the catheter has not moved after initial insertion. Metric markings on the shunt catheters in the operating room would be very helpful in determining the final position of the catheter, as the catheter is likely to move during the process of cutting the ventricular catheter to an appropriate length and connecting the intracranial catheter to the proximal shunt valve.
The distribution of delta measurements of both shunt and EVD catheter placements were plotted on a frequency plot [
Out of the 217 EVDs inserted during this time, 21 of these needed to be revised and replaced. Out of the 60 shunts inserted, 6 of them needed a shunt revision. There was not enough power to analyze if there was an effect on revision or replacement rates. While it would be interesting to see if there was a difference in revision rates due to discrepancy in the length of catheter insertion, there are still a variety of factors that would influence this, including contact with the ventricular walls, 3rd ventricle catheter placement, postinsertion hemorrhage, and valve/distal catheter malfunction.[
At the start of our study, we intended to analyze the effect of intraoperative neuronavigation on the accuracy of catheter placement. However, the use of this technology has been a recent addition to our institution’s practice in 2020. Ten shunts were placed using intraoperative navigation, and no EVDs were placed with neuronavigation. Given that only a small percentage of total shunts were placed with navigation, we were unable to apply meaningful statistics. There is a growing literature in the realm of the accuracy of ventricular catheter placement using navigation technology. However, the definition of accuracy is varied throughout this literature. Examples include the tip of the catheter within the intended ventricle, Euclidean distance from the ipsilateral foramen of Monro, or a grading system described by Hayhurst et al. qualitatively describing the tip of the catheter to its surrounding anatomy.[
Limitations
One of the major limitations of this study was that there was a discrepancy between the number of subjects in the EVD group compared to the shunt group. The smaller sample size of shunt catheters measured could have biased our mean delta in the shunt group toward greater variance from the intended depth of insertion. Another limitation of our study is that this is a retrospective study, which may lead to missing information as the catheters were not placed with this study in mind. It also introduces confounding variables such as hospital course that was not able to be controlled at the time of study. In addition, this is a single institution study, which may limit our generalizability to a larger population; however, other institutions similarly place their catheters and utilize the same type of catheter.
Generalizability
This smaller sample size may limit the generalizability of this study; however, because our sample included a variety of individuals with a large age range, our results should be applicable to individuals who receive intracranial catheters for a variety of reasons.
Interpretation
The problem of not having continuous markings of depth that the user easily interprets is not limited to ventricular catheters. There are no markings on a much longer intrathecal catheter used for intrathecal pumps (Medtronic Inc.). Precise drug delivery into the intrathecal space depends on knowing the exact length of the catheter inserted and is prone to errors in administering faulty amounts of medication. The Tuohy needle used for epidural anesthesia does not have centimeter markings that are easily interpreted by the user and involves converting the shaded parts of the needle to depth.
CONCLUSION
Although this is a simple change in the design, the addition of metric unit markings by the manufacturer would greatly assist surgeons in assessing the depth of catheter insertion and reduce malfunction as well as potential neurovascular injury to surrounding structures.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study. Our institution approved this as an exempt study on 9/12/2022 in exemption category 4.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Borgbjerg BM, Gjerris F, Albeck MJ, Hauerberg J, Børgesen SE. Frequency and causes of shunt revisions in different cerebrospinal fluid shunt types. Acta Neurochir (Wien). 1995. 136: 189-94
2. Browd SR, Ragel BT, Gottfried ON, Kestle JR. Failure of cerebrospinal fluid shunts: Part I: Obstruction and mechanical failure. Pediatr Neurol. 2006. 34: 83-92
3. Buster BE, Bonney PA, Cheema AA, Glenn CA, Conner AK, Safavi-Abbasi S. Proximal ventricular shunt malfunctions in children: Factors associated with failure. J Clin Neurosci. 2016. 24: 94-8
4. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, editors. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. p. 6-15
5. Dobran M, Nasi D, Mancini F, Gladi M, Polonara G, Marini A. Relationship between the location of the ventricular catheter tip and the ventriculoperitoneal shunt malfunction. Clin Neurol Neurosurg. 2018. 175: 50-3
6. Hayhurst C, Byrne P, Eldridge PR, Mallucci CL. Application of electromagnetic technology to neuronavigation: A revolution in image-guided neurosurgery. J Neurosurg. 2009. 111: 1179-84
7. Institute of Medicine (US) Committee on Quality of Health Care in AmericaKohn LT, Corrigan JM, Donaldson MS, editors. To err is human: Building a safer health system. Washington, DC: National Academies Press (US); 2000. p.
8. Levitt MR, O’Neill BR, Ishak GE, Khanna PC, Temkin NR, Ellenbogen RG. Image-guided cerebrospinal fluid shunting in children: Catheter accuracy and shunt survival. J Neurosurg Pediatr. 2012. 10: 112-7
9. Reddy GK. Ventriculoperitoneal shunt surgery and the incidence of shunt revision in adult patients with hemorrhage-related hydrocephalus. Clin Neurol Neurosurg. 2012. 114: 1211-6
10. Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ. Mechanical complications in shunts. Pediatr Neurosurg. 1991. 17: 2-9
11. Stein SC, Guo W. Have we made progress in preventing shunt failure? A critical analysis. J Neurosurg Pediatr. 2008. 1: 40-7
12. Vlasak A, Okechi H, Horinek D, Albright AL. Pediatric ventriculoperitoneal shunts revision rate and costs in high-volume sub-Saharan department. World Neurosurg. 2019. 130: e1000-3
13. Wiklund M, editors. Eleven keys to designing error-resistant medical device. Medical Device and Diagnostic Industry;. 2002. p.
14. Wilson TJ, Stetler WR, Al-Holou WN, Sullivan SE. Comparison of the accuracy of ventricular catheter placement using freehand placement, ultrasonic guidance, and stereotactic neuronavigation. J Neurosurg. 2013. 119: 66-70
15. Yamada SM, Kitagawa R, Teramoto A. Relationship of the location of the ventricular catheter tip and function of the ventriculoperitoneal shunt. J Clin Neurosci. 2013. 20: 99-101