- Department of Neurological Surgery, University of Alabama at Birmingham, Birmingham, Alabama,
- Department of Neurosurgery, Westchester Medical Center, Valhalla, New York, United States,
- Departments of Radiology, Rutgers - Robert Wood Johnson Medical School, New Brunswick, Canada.
Correspondence Address:
Gaurav Gupta
Department of Neurological Surgery, University of Alabama at Birmingham, Birmingham, Alabama,
DOI:10.25259/SNI_160_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Matthew Parr1, Nitesh Patel1, John Kauffmann1, Fawaz Al-Mufti2, Sudipta Roychowdhury3, Vinayak Narayan1, Michael Nosko1, Anil Nanda1, Gaurav Gupta1. Arteriovenous malformation presenting as traumatic subdural hematoma: A case report. 25-Jul-2020;11:203
How to cite this URL: Matthew Parr1, Nitesh Patel1, John Kauffmann1, Fawaz Al-Mufti2, Sudipta Roychowdhury3, Vinayak Narayan1, Michael Nosko1, Anil Nanda1, Gaurav Gupta1. Arteriovenous malformation presenting as traumatic subdural hematoma: A case report. 25-Jul-2020;11:203. Available from: https://surgicalneurologyint.com/surgicalint-articles/10163/
Abstract
Background: Brain arteriovenous malformations (AVMs) are congenital aberrant connections between afferent arteries and draining veins with no intervening capillary bed or neural parenchyma. Other than seizures, the most common initial presentation of AVM is hemorrhage, which is typically intraparenchymal, subarachnoid, or intraventricular, and very rarely subdural.
Case Description: This patient is a 66-year-old male with a history of atrial fibrillation, chronically anticoagulated with apixaban, who presented through emergency services after a fall. On presentation, computed tomography (CT) of the head showed a small, 6 mm right subdural hematoma, and the patient was neurologically intact. The hematoma was evacuated by burr hole craniotomy and placement of a subdural drain 12 days after the initial presentation due to worsening headaches and further hematoma expansion. Two weeks postevacuation, the patient was readmitted for seizures, and at this time, CT angiography showed no intracranial vascular lesion. Approximately 1 month later, the patient was readmitted for decreased responsiveness, and CT head at this time found right frontal intraparenchymal hemorrhage. On subsequent catheter angiography, the right frontal AVM was discovered. It was treated with preoperative embolization followed by surgical resection. Postoperatively, the patient followed commands and tracked with his eyes. There was spontaneous antigravity movement of the right upper extremity, but still no movement of the left upper or bilateral lower extremities.
Conclusion: This case emphasizes the importance of maintaining a high index of suspicion for underlying vascular lesions when evaluating intracranial bleeding, even in the setting of traumatic history, particularly in cases of hematoma expansion.
Keywords: Arteriovenous malformation, Intracranial hemorrhage, Subdural hematoma
INTRODUCTION
Brain arteriovenous malformations (AVMs) are congenital aberrant connections between afferent arteries and draining veins with no intervening capillary bed or neural parenchyma. These are distinct from other cerebral vascular anomalies such as cavernous malformations and dural AV fistulas.[
The most common initial symptomatic presentation of AVM is hemorrhage, which is typically intraparenchymal, subarachnoid, or intraventricular, but less frequently can be subdural.[
In the following case, we present a patient in whom the diagnosis of AVM was missed on initial presentation for subdural hematoma (SDH), which was believed to be secondary to a traumatic etiology. The AVM was later diagnosed by catheter angiography after rupture and intraparenchymal bleed.
CASE REPORT
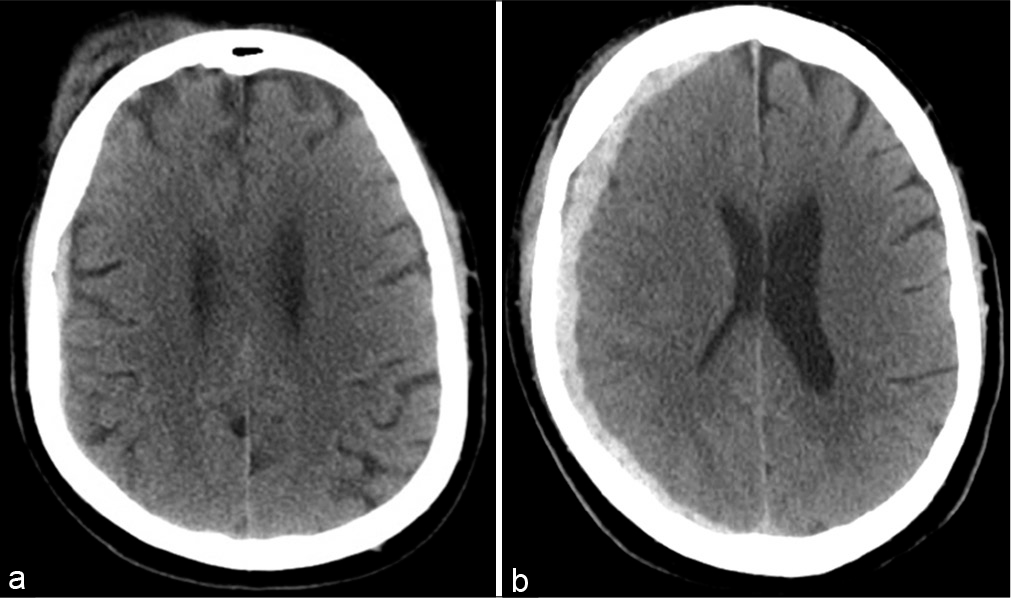
This is a 66-year-old male with a medical history significant for hypertension, hyperlipidemia, hepatitis C virus infection, and atrial fibrillation chronically anticoagulated with apixaban. He was brought in by emergency services after a fall. The patient reported dizziness, then lost consciousness, and was found down by a family member. Primary trauma survey was negative with a Glasgow Coma Score of 15 no focal neurological deficits. Noncontrast CT of the head [
The patient was admitted to the surgical intensive care unit for serial checks. On the 2nd hospital day, he developed severe headache, and the head CT was repeated [
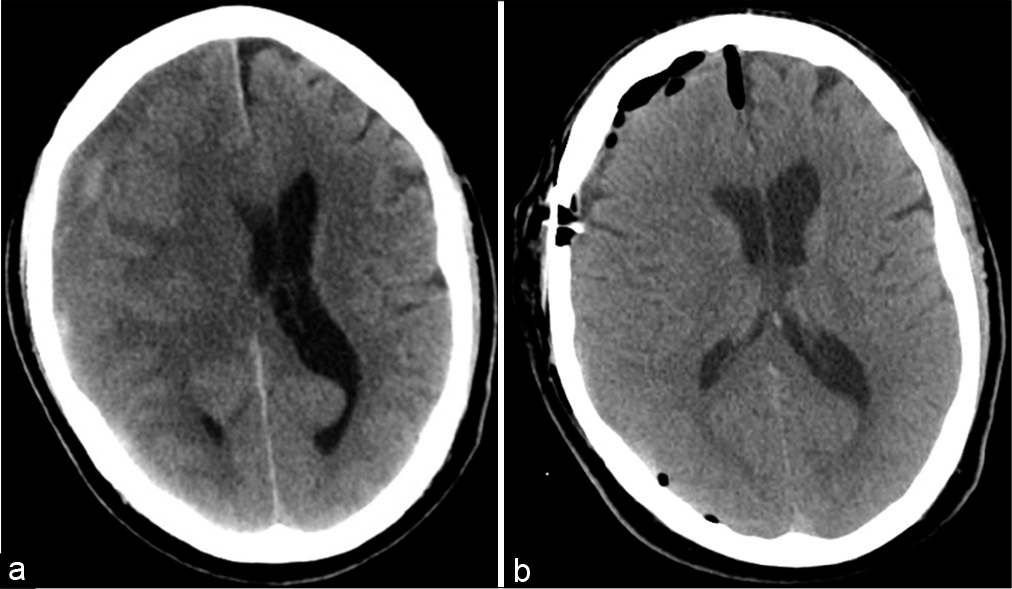
In rehabilitation, 6 days after discharge, the patient experienced a terrible headache for 3 days, which prompted a repeat neurosurgical evaluation. The patient was still neurologically intact at this time, and repeat CT head [
On the 14th postoperative day, the patient was seen in the office for follow-up. The patient described a strange sensation in the right arm, as well as the feeling that he could not control the arm. Based on these symptoms, he was sent directly to the emergency room for further evaluation. In the emergency room, the patient developed a focal left arm seizure. He was treated for this seizure and started on electroencephalography (EEG) monitoring. He was then admitted. That night, the patient was found to be unresponsive; CT head showed no acute hemorrhage and EEG indicated status epilepticus. The patient was intubated for airway protection and transferred to the medical intensive care unit for further management.
As the patient’s initial presentation was hemorrhagic but thought to be traumatic, dedicated vascular imaging had not been obtained previously. During this third admission, cerebral CTA was obtained as his clinical course became suspicious for either an underlying lesion or vascular issue. The studies were negative for any pathology. This admission was complicated by ventilator-dependent respiratory failure, septic shock secondary to Pseudomonas bacteremia, and the patient received a tracheostomy and gastrostomy. The patient was eventually stabilized and discharged to long-term acute care hospital on day 29 with levetiracetam and oxcarbazepine for seizure control.
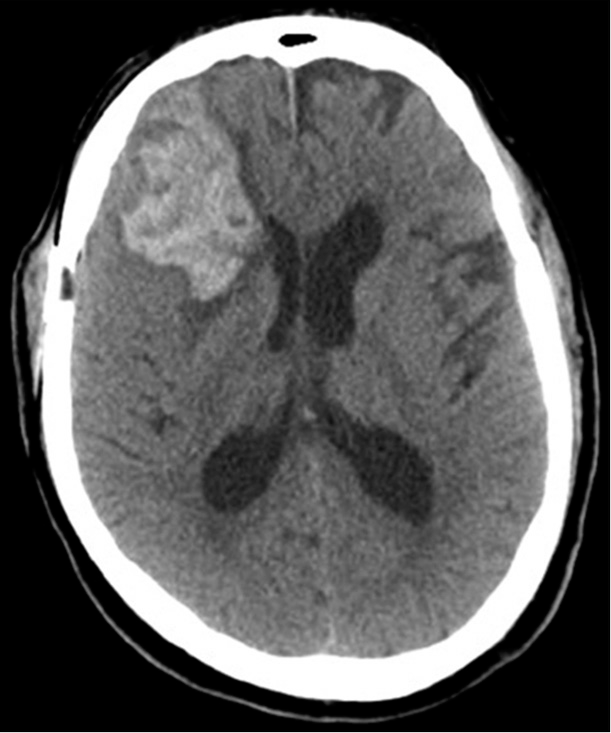
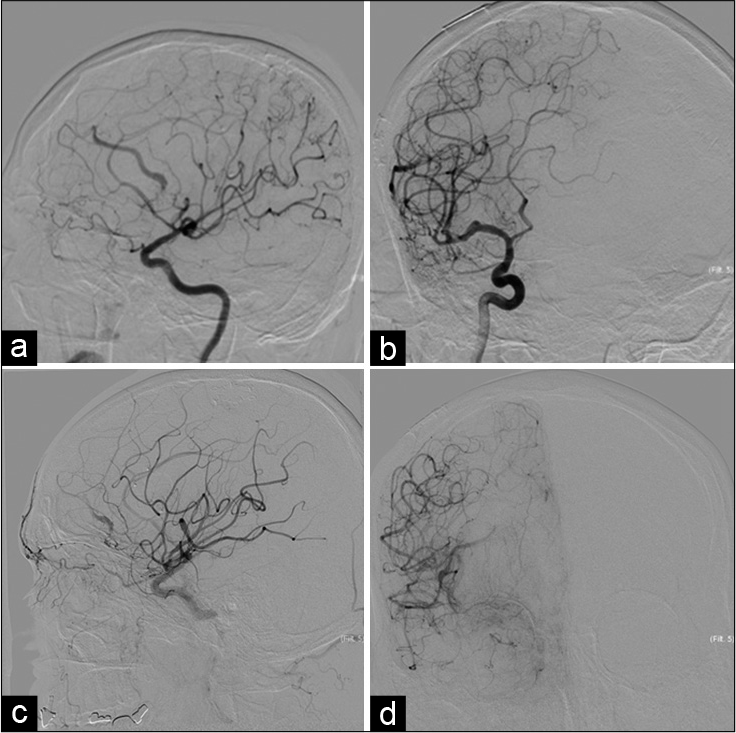
Four days after discharge, the patient was found to be less responsive and was transferred back for neurosurgical evaluation. The patient opened eyes to stimulation, localized with the right upper extremity, and had minimal movement of left upper and bilateral lower extremities, and cough, gag, corneal, and pupillary reflexes were all present. CT and CTA head [
On the 8th day postembolization, the patient was taken to the operating room for resection of the AVM. The right pterional craniotomy was performed to access the AVM, which was separated from surrounding brain parenchyma using microdissection techniques. The feeding arteries, followed by the draining vein, were coagulated using bipolar electrocautery and resected. Following resection, intraoperative Doppler ultrasound indicated no arterial flow in the draining vein. The associated intraparenchymal hematoma was also evacuated. Postoperative angiography showed no residual AVM.
Postoperatively, the patient’s mental status progressively improved. On the 3rd postoperative day, the patient followed commands and tracked with his eyes. There was spontaneous antigravity movement of the right upper extremity, but still no movement of the left upper or bilateral lower extremities. The postoperative course was complicated by pseudomonas sepsis, which was treated in consultation with infectious disease. The patient was eventually discharged to long-term acute care on the 15th postoperative day.
DISCUSSION
In this report, we present a case of an AVM, which ruptured, leading to intraparenchymal hemorrhage. This patient’s. initial presentation was 2 months before the rupture, when he was brought to the ED with a SDH after falling from standing, while systemically anticoagulated. Due to the traumatic etiology of presentation, angiographic studies were not conducted at this time, and the AVM was not recognized. The SDH eventually required evacuation, which was uneventful, and again did not reveal the AVM. The patient subsequently was readmitted for seizures. CTA at this time did not reveal the AVM. Approximately 1 month later, at the time that the AVM ruptured and the patient suffered intraparenchymal hemorrhage, the AVM was diagnosed by catheter angiography. The AVM was subsequently treated by staged endovascular embolization and open surgical resection. This case showed that what might appear as a simple traumatic hematoma may in fact be a more subtle pathology. Further, when a known hematoma continues to expand without any other cause, or a SDH is identified without a traumatic etiology, a vascular lesion should be considered in the differential.
AVMs are aberrant connections between afferent arteries and draining veins with no intervening capillary bed.[
Overall, the risk of spontaneous hemorrhage of an AVM is 2–4% per year, amounting to approximately 78% lifetime risk in the instance, for example, of an annual risk of 3% and a remaining life expectancy of 50 years, but this number is dependent on the remaining life expectancy of the patient.[
In the instance of the patient discussed above, it is unknown whether the initial SDH was caused by rupture of the AVM, as the hematoma was overlying the AVM, but occurred in the setting of trauma with systemic anticoagulation. In a traumatic presentation of SDH, it is not typical to utilize angiographic imaging. In a study of 600 traumatic brain injury patients, Naraghi et al. found that 132 (22%) underwent CTA, but management was only changed in one patient after CTA, who then underwent catheter angiography, which ended up being negative. They concluded that CTA is not necessary in the initial evaluation of most trauma patients.[
In this particular case, it cannot be known if the initial SDH could be referable to the later discovered AVM, or if it was simply the sequelae of trauma. Regardless, one should remain suspicious of an underlying vascular lesion when evaluating the imaging of any intracranial hemorrhage. Even if vascular imaging is not initially indicated in the setting of traumatic etiology, this suspicion should be maintained.
The patient later experienced headaches, which was attributed to expansion of the hematoma, but the AVM may have also been a contributing factor. When the patient represented with seizure, CTA was conducted, but was unable to detect the AVM at this time. While other angiographic modalities, such as CT and MR angiography, are commonly utilized as initial studies, as they are less invasive imaging options, conventional catheter angiography is the gold standard for the diagnosis of AVM. Earlier angiography, particularly when the patient experienced headache in the subacute phase of the initial SDH, could have resulted in earlier diagnosis of this patient’s AVM, possibly avoiding the eventual intraparenchymal hemorrhage.
CONCLUSION
Above, we discuss a case of AVM in which the diagnosis was not made on initial presentation for acute SDH secondary to what was believed to be a traumatic etiology. This AVM later ruptured leading to intraparenchymal hemorrhage. This case reinforces the importance of maintaining a high index of suspicion for underlying vascular lesions when evaluating intracranial bleeding, particularly in cases of hematoma expansion, even in the setting of trauma.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abecassis IJ, Xu DS, Batjer HH, Bendok BR. Natural history of brain arteriovenous malformations: A systematic review. Neurosurg Focus. 2014. 37: E7
2. Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC. Prospective, population-based detection of intracranial vascular malformations in adults: The scottish intracranial vascular malformation study (SIVMS). Stroke. 2003. 34: 1163-9
3. Aoun SG, Bendok BR, Batjer HH. Acute management of ruptured arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012. 23: 87-103
4. Atkinson RP, Awad IA, Batjer HH, Dowd CF, Furlan A, Giannotta SL. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001. 32: 1430-42
5. Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Elliott JP. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999. 178: 517-22
6. Choi JH, Mast H, Sciacca RR, Hartmann A, Khaw AV, Mohr JP. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke. 2006. 37: 1243-7
7. Gabriel RA, Kim H, Sidney S, McCulloch CE, Singh V, Johnston SC. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke. 2010. 41: 21-6
8. Goldberg J, Raabe A, Bervini D. Natural history of brain arteriovenous malformations: Systematic review. J Neurosurg Sci. 2018. 62: 437-43
9. Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983. 58: 331-7
10. Hartmann A, Mast H, Mohr JP, Koennecke HC, Osipov A, Pile-Spellman J. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke. 1998. 29: 931-4
11. Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: A long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008. 63: 823-9
12. Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001. 95: 633-7
13. Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995. 37: 851-5
14. Laakso A, Reza D, Seppanen J, Juvela S, Vaart K, Niemela M. Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery. 2008. 63: 244-55
15. Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet. 2014. 383: 614-21
16. Mossa-Basha M, Chen J, Gandhi D. Imaging of cerebral arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012. 23: 27-42
17. Naraghi L, Larentzakis A, Chang Y, Duhaime AC, Kaafarani H, Yeh DD. Is CT angiography of the head useful in the management of traumatic brain injury?. J Am Coll Surg. 2015. 220: 1027-31
18. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986. 65: 476-83
19. Spetzler RF, Ponce FA. A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J Neurosurg. 2011. 114: 842-9
20. Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP. The new york islands AVM study: Design, study progress, and initial results. Stroke. 2003. 34: e29-33
21. Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES. Predictors of hemorrhage in patients with untreated brain arteriovenous malformations. Neurology. 2006. 66: 1350-5
22. Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus. 2010. 29: E7