- Department of Neurological Surgery, Henry Ford Hospital, 2799 W. Grand Blvd, Detroit, MI 48202, USA
Correspondence Address:
Aqueel H. Pabaney
Department of Neurological Surgery, Henry Ford Hospital, 2799 W. Grand Blvd, Detroit, MI 48202, USA
DOI:10.4103/2152-7806.179579
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pabaney AH, Ali R, Kole M, Malik GM. Arteriovenous malformations of the corpus callosum: Pooled analysis and systematic review of literature. Surg Neurol Int 01-Apr-2016;7:
How to cite this URL: Pabaney AH, Ali R, Kole M, Malik GM. Arteriovenous malformations of the corpus callosum: Pooled analysis and systematic review of literature. Surg Neurol Int 01-Apr-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/arteriovenous-malformations-of-the-corpus-callosum-pooled-analysis-and-systematic-review-of-literature/
Abstract
Background:Arteriovenous malformations (AVMs) of the corpus callosum (CC) are rare entities. We performed a systematic review of the available literature to better define the natural history, patient characteristics, and treatment options for these lesions.
Methods:A MEDLINE, Google Scholar, and The Cochrane Library search were performed for studies published through June 2015. Data from all eligible studies were used to examine epidemiology, natural history, clinical features, treatment strategies, and outcomes of patients with CC-AVMs. A systematic review and pooled analysis of the literature were performed.
Results:Our search yielded 37 reports and 230 patients. Mean age at presentation was 26.8 years (±13.12 years). AVMs were most commonly located in the splenium (43%), followed by the body (31%), and then the genu (23%) of the CC. A Spetzler-Martin grade of III was the most common (37%). One hundred eighty-seven (81.3%) patients presented with hemorrhage, 91 (40%) underwent microsurgical excision, and 87 (38%) underwent endovascular embolization. Radiosurgery was performed on 57 (25%) patients. Complete obliteration of the AVM was achieved in 102 (48.1%) patients and approximately twice as often when microsurgery was performed alone or in combination with other treatment modalities (94% vs. 49%; P P = 0.35).
Conclusion:We present an analysis of the pooled data in the form of a systematic review focusing on management of CC-AVMs. This review aims to provide a valuable tool to aid in decision making when dealing with this particular subtype of AVM.
Keywords: Arteriovenous malformation, corpus callosum, endovascular therapy
INTRODUCTION
Arteriovenous malformations (AVMs) of the corpus callosum (CC) are distinct clinical as well as surgical entities. They are known to cause recurrent hemorrhage more frequently as compared to more superficial, pial AVMs.[
METHODS
We undertook a review of the literature on CC-AVMs. Our methods were in accordance with the systematic review section of the preferred reporting items for systematic reviews and meta-analyses statement.[
Search strategy
Two independent reviewers (Rushna Ali and Aqueel H. Pabaney) performed a comprehensive electronic search for articles on CC-AVMs. The search included MEDLINE (PubMed and Ovid), Google Scholar, and The Cochrane Library from January 1970 to June 2015. These databases were queried using keywords and MeSH terms “arteriovenous malformation,” “AVM,” “corpus callosum,” “radiosurgery,” “endovascular therapy,” “microsurgery,” “surgical resection,” and “outcome.” A full-text version was obtained for all studies the reviewers considered relevant. To identify additional resources, we manually searched references of articles that could be potentially relevant. No restrictions were imposed based on publication dates, types, or language.
Study selection
We selected articles reporting clinical outcomes for patients with CC-AVMs treated with any of the three treatment modalities (i.e., microsurgery, endovascular embolization, and RS). We included articles that documented patient age, clinical presentation, location of AVM, treatment rendered, and outcomes (modified Rankin Scale [mRS] or posttreatment complications). We included studies not published in English if they met the above selection criteria. We excluded articles that did not report the location of AVM, treatment modality, or outcomes as well as abstracts and letters to the editor that were not published as full reports. Disagreements between the two reviewers about the relevance of a particular study were resolved by discussion and consensus with a third reviewer (Ghaus M. Malik or Maximillian Kole).
Data extraction
Two independent reviewers (Rushna Ali and Aqueel H. Pabaney) performed data extraction. Discrepancies were settled by discussion and consensus. Information was gathered from eligible articles (systematic review) using data abstraction forms, which included the following information: Total number of patients, patients’ age and gender, location of the AVM within the CC as defined by Yasargil's classification,[
Definition of variables
AVM location was divided into those present in the genu, body, or splenium of the CC. Presenting symptoms were categorized as hemorrhage (i.e., subarachnoid [SAH], intraventricular, and intracerebral [ICH]), seizures, headaches, and neurological deficits. Others were classified as incidental findings. Treatment strategy was divided into microsurgical resection, endovascular embolization, radiation therapy, or a combination of two or more strategies. Obliteration rates were defined as either complete obliteration or residual AVM. Long-term outcome was defined by mRS or postoperative complications.
Statistical analysis
We used an independent two-tailed t-test (Welch generalization of the Student's t-test, Microsoft Excel, 2013, Redmond, WA, USA) to compare preoperative and postoperative mRSs. Chi-square test was utilized to compare the difference in AVM obliteration rates achieved by various treatment modalities. P value of 0.05 was considered statistically significant.
RESULTS
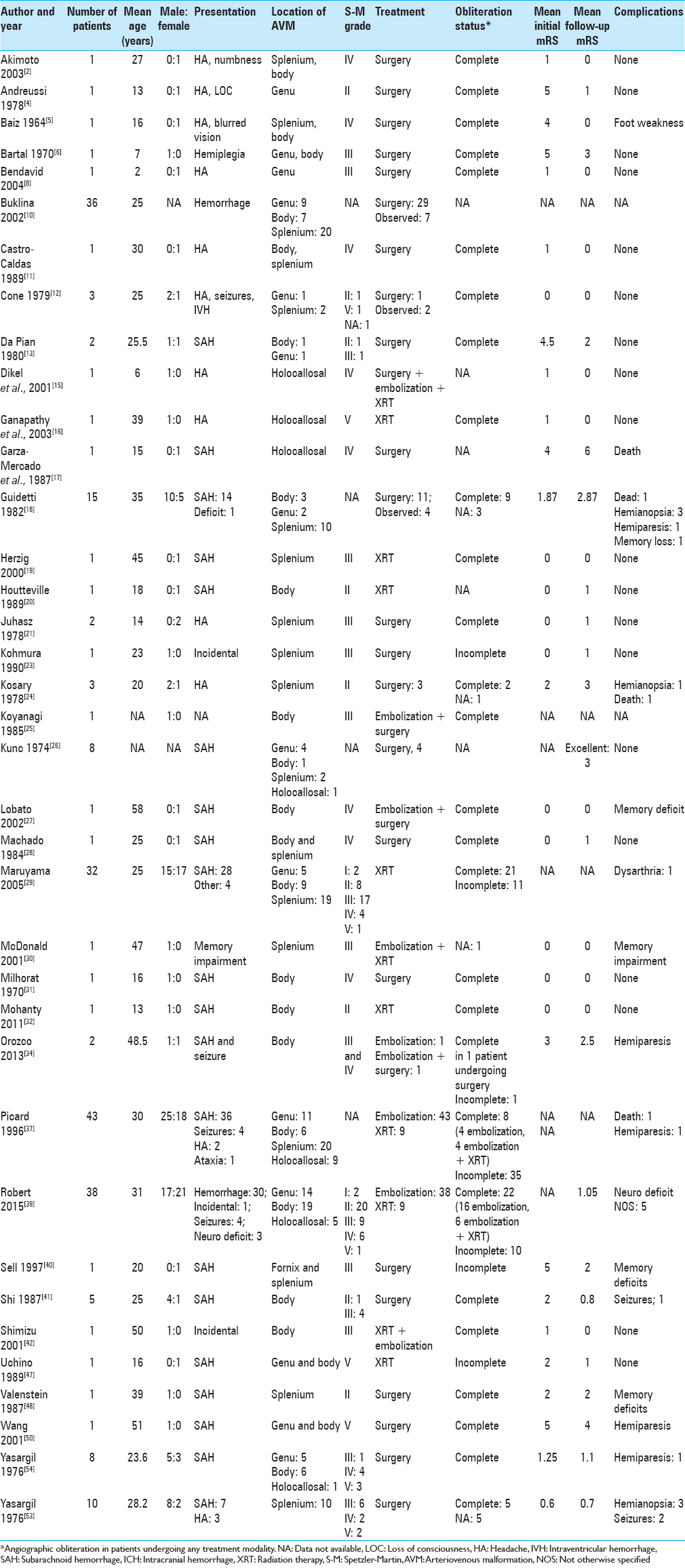
Eligibility criteria described in the methods section were met by 37 articles, with a total of 230 patients [
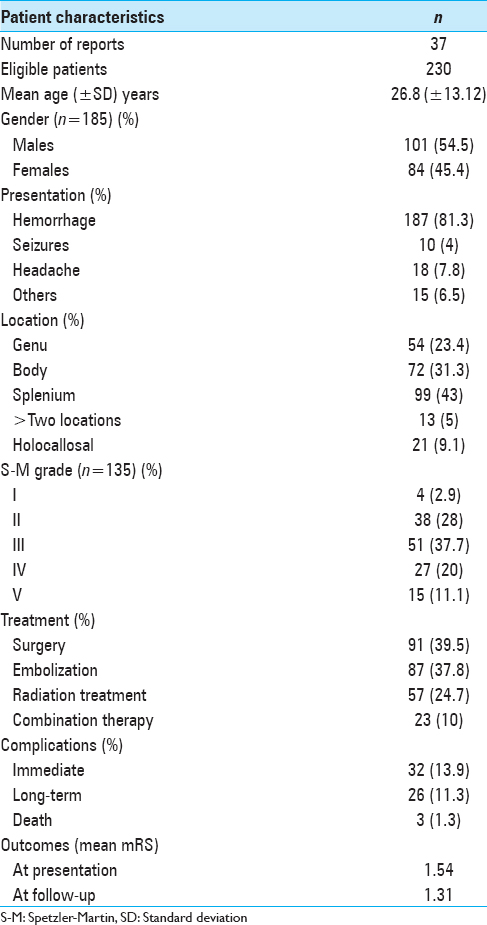
The mean patient age at presentation was 26.82 years (±13.12 years; range 2–61 years; median 25 years) with a slight male (55%) preponderance. CC-AVMs were most commonly located in the splenium (99 patients; 43%), followed by the body (72 patients; 31%), and then the genu (54 patients; 23%). Two or more areas of CC were involved in 13 patients (6%) whereas the entire CC was involved in 21 (9%). Spetzler-Martin grade was reported or could be calculated for 135 patients. The most common Spetzler-Martin grade was 3 (51 patients; 37%) followed by 2 (38 patients; 28%).
Overall, analysis of 230 patients showed that 187 (81.3%) presented with hemorrhage, 5 with focal neurological deficit, 10 with seizures without hemorrhage, and 18 with headaches as the primary symptom. Ninety-one (40%) patients underwent microsurgical resection of the AVM. Endovascular embolization was performed either as a preoperative adjunct or as a stand-alone treatment modality in 87 (38%) patients. RS was administered to 57 (25%) patients while two or more treatment modalities were used in combination in 23 (10%). Cumulatively, 212 (92.1%) patients received treatment whereas 18 (7.8%) were managed conservatively. Complete obliteration of the AVM was achieved in 102 (48.1%) patients. This was accomplished by microsurgery in 44 (43.1%) patients, embolization in 20 (19.6%), RS in 24 (23.5%), and combination therapy in 14 (13.7%).
Residual AVM was observed in 60 (28.3%) patients. Half of these patients underwent stand-alone embolization (30 patients; 50%), 12 (20%) RS alone, and 15 (25%) received combination therapy of embolization and RS. Of those who underwent surgical resection, only 3 (5%) patients had a residual nidus. Stand-alone surgical resection or in combination with other treatment modalities resulted in complete obliteration in 47 of 50 (94%) patients whereas only 55 (49%) of 112 who underwent embolization alone, RS alone, or combination therapy with embolization and RS achieved complete obliteration. This difference was found to be statistically significant (P < 0.001). Thirty-two patients encountered complications in the immediate posttreatment period; however, only 26 patients were left with long-lasting adverse effects from treatment. Three deaths were noted in the literature, 2 occurred posttreatment, and 1 patient died after managed conservatively. Mean mRS at presentation was 1.54 and mean mRS at last follow-up was 1.31. The difference between the initial and final mRS was not statistically significant (P = 0.35) [
DISCUSSION
Epidemiology and natural history
CC-AVMs are relatively rare. The literature is only populated with case series and case reports; hence, the natural history of these lesions remains largely unknown. The prevalence of CC-AVMs ranges from 1.1% to 3% in population-based studies[
Anatomical considerations
CC-AVMs are generally supplied by the branches of the anterior cerebral artery and the posterior cerebral artery (PCA) with minor contributions from the middle cerebral artery. A recent study indicated that 27 (59%) of the 46 nidi studied were supplied by both anterior and posterior circulations and 30 (65%) of 46 nidi were fed by bilateral pericallosal arteries.[
Anatomically, CC-AVMs can be divided into four main groups as described by Yasargil et al.[
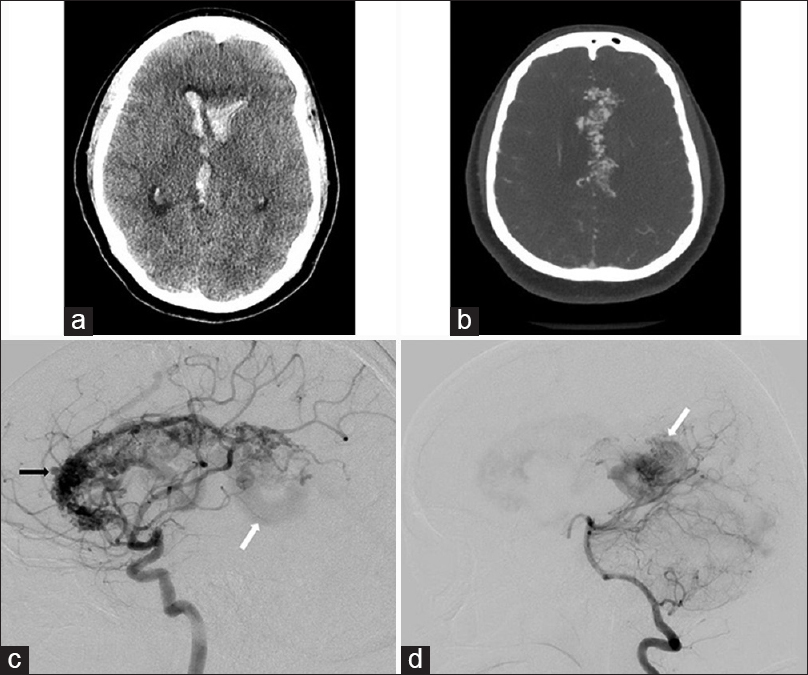
Figure 1
(a) Axial noncontrast computed tomography scan obtained on a young female patient presenting to our institution with headaches and altered mental status shows intraventricular hemorrhage. (b) Axial computed tomography angiogram performed on the same patient reveals an extensive arteriovenous malformation involving the entire corpus callosum (holocallosal). (c) Lateral projection of internal carotid artery injection reveals a large arteriovenous malformation involving the genu, rostrum, and body of the corpus callosum. Intranidal aneurysm is also appreciated (black arrow). Early venous drainage is seen via galenic system (white arrow). (d) Lateral projection of vertebral artery injection reveals filling of the splenial component of the arteriovenous malformation nidus not seen with internal carotid artery injection (white arrow)
Picard et al. further divided the AVM nidi into three types such as (1) the “compact” nidus, which is well demarcated and located within the CC; (2) an “extensive” nidus involving the cingulate gyrus or septum pellucidum in addition to the CC; and (3) a “diffuse” nidus which is ill-defined and involves various cortical, subcortical, and intraventricular regions.[
Our analysis reveals that the most common location for CC-AVMs is the splenium. Approximately 10% of all CC-AVMs involve the white matter structure in its entirety, and these are the most difficult to treat.
Treatment
Multimodal treatment of complex AVMs is advocated. Microsurgical resections aided by endovascular embolization and stereotactic RS are the cornerstones of therapy for any AVM. Although advances in microsurgery have made complete resection of these “inoperable” lesions possible, the cure comes with a considerable risk of developing new neurological deficits. Endovascular embolization rarely achieves complete obliteration of the AVM but can significantly aid in occluding feeders that are difficult to access early in surgery. Utilization of RS for deep-seated AVMs with nidi measuring <3 cm3 in volume has increased significantly over the last two decades with encouraging results. A more detailed discussion of these treatment modalities is as follows.
Surgical resection
Historically, surgical resection of CC-AVMs has been fraught with significant morbidity. However, with advanced microsurgical techniques and widespread availability of surgical adjuncts such as preoperative endovascular embolization and radio surgical downgrading, better results are seen in the more recent literature. One of the earliest series of surgical resection of CC-AVMs was published by Yasargil et al., in 1976. Eighteen patients were treated with microsurgical resection without preoperative embolization or radiotherapy.[
Bartal and Yahel argue that the incidence of paraventricular lesions, including CC-AVMs, is underestimated and needs more attention as they have a higher propensity to bleed and surgical resection could result in establishing a definitive cure.[
Our pooled analysis of the literature identified fifty patients who underwent surgical resection of the CC-AVM with or without prior embolization or RS, and cure was achieved in 47 (94%). Only 2 of 46 patients who underwent stand-alone surgical excision of the AVM had residual AVM on follow-up angiogram. Sixteen (32%) patients developed permanent complications after surgery, and only 1 patient died after surgical intervention.
Endovascular therapy
CC-AVMs are difficult to treat with endovascular therapy alone. The goal of embolization should be prevention of hemorrhage and a decrease in nidus size to facilitate subsequent surgical resection or RS. In 1996, Picard et al. described a series of AVMs involving the CC that were treated by endovascular means only.[
Radiosurgery
RS is increasingly employed for treatment of small (<3 cm3) as well as some medium- (3–6 cm) and large-size (>6 cm3) AVMs located in deep cerebral tissue. RS is an attractive choice due to its noninvasive nature, minimal risk for acute complications, and shorter recovery time for the patients. However, cure is not immediate and can take 2–3 years for the effects of radiation to result in thrombosis of the AVM. Although smaller AVMs (<3 cm3) are ideal candidates for RS, several reports have confirmed lower obliteration rate in larger AVMs.[
CONCLUSIONS
There is a paucity of available literature on CC-AVMs. The appropriate treatment strategy for these rare lesions remains controversial. Through this systematic review of literature and analysis of pooled data encompassing almost half a century, the authors have attempted to provide a tool that will assist healthcare providers to formulate an individualized treatment plan for these patients. Although microsurgical resection offers the most definitive treatment of these lesions, improved outcomes have been observed when microsurgery is supplemented with advances in RS and endovascular therapy. However, the decision to treat a patient with CC-AVM should weigh the natural history of these lesions against potential neurological and neuropsychological morbidity that may follow treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abla AA, Rutledge WC, Seymour ZA, Guo D, Kim H, Gupta N. A treatment paradigm for high-grade brain arteriovenous malformations: Volume-staged radiosurgical downgrading followed by microsurgical resection. J Neurosurg. 2015. 122: 419-32
2. Akimoto H, Komatsu K, Kubota Y. Symptomatic de novo arteriovenous malformation appearing 17 years after the resection of two other arteriovenous malformations in childhood: Case report. Neurosurgery. 2003. 52: 228-31
3. Al-Shahi R, Fang JS, Lewis SC, Warlow CP. Prevalence of adults with brain arteriovenous malformations: A community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry. 2002. 73: 547-51
4. Andreussi L, Borella F, Cama A, Marino C. Microsurgical excision of an arteriovenous malformation of the anterior corpus callosum. Surg Neurol. 1978. 10: 97-9
5. Baiz TC, Jakoby RK. Surgical treatment of an arteriovenous malformation associated with agenesis of the corpus callosum. J Neurosurg. 1964. 21: 306-8
6. Bartal AD, Yahel MA. Total excision of an arteriovenous malformation of the corpus callosum. Case report. J Neurosurg. 1970. 33: 95-9
7. Bassett RC. Surgical experiences with arteriovenous anomalies of the brain. J Neurosurg. 1951. 8: 59-74
8. Bendavid OJ, Khoshyomn S, Wilson JT. Arteriovenous malformation of the genu of the corpus callosum. Pediatr Neurosurg. 2004. 40: 49-50
9. Buklina SB. The corpus callosum, interhemisphere interactions, and the function of the right hemisphere of the brain. Neurosci Behav Physiol. 2005. 35: 473-80
10. Buklina SB. The unilateral spatial neglect phenomenon in patients with arteriovenous malformations of deep brain structures. Neurosci Behav Physiol. 2002. 32: 555-60
11. Castro-Caldas A, Poppe P, Antunes JL, Campos J. Partial section of the corpus callosum: Focal signs and their recovery. Neurosurgery. 1989. 25: 442-7
12. Cone JD, Maravilla KR, Cooper PR, Diehl JT, Clark WK. Computed tomography findings in ruptured arteriovenous malformations of the corpus callosum. J Comput Assist Tomogr. 1979. 3: 478-82
13. Da Pian R, Pasqualin A, Scienza R, Vivenza C. Microsurgical treatment of ten arteriovenous malformations in critical areas of the cerebrum. J Microsurg. 1980. 1: 305-20
14. Dawson RC, Tarr RW, Hecht ST, Jungreis CA, Lunsford LD, Coffey R. Treatment of arteriovenous malformations of the brain with combined embolization and stereotactic radiosurgery: Results after 1 and 2 years. AJNR Am J Neuroradiol. 1990. 11: 857-64
15. Dikel TN, Fennell EB, Nadeau SE, Quisling RG, Mickle JP, Friedman WA. A neuropsychological outcome study of a child's left pericallosal arteriovenous malformation with occult fornix lesion. Neurocase. 2001. 7: 503-13
16. Ganapathy K, Shankarnarayanan V, Saji , Padmanabhan TK, Halbe S. Obliteration of giant corpus callosum AVM with linac based stereotactic radiosurgery. J Clin Neurosci. 2003. 10: 272-6
17. Garza-Mercado R, Cavazos E, Tamez-Montes D. Cerebral arteriovenous malformations in children and adolescents. Surg Neurol. 1987. 27: 131-40
18. Guidetti B, Spallone A. The management of arteriovenous malformations of the corpus callosum. Neurol Res. 1982. 4: 253-82
19. Herzig R, Burval S, Vladyka V, Janouskova L, Krivanek P, Krupka B. Familial occurrence of cerebral arteriovenous malformation in sisters: Case report and review of the literature. Eur J Neurol. 2000. 7: 95-100
20. Houtteville JP, el Omeiri S, Derlon JM, Toumi K, Benazza A. Arteriovenous aneurysm of the corpus callosum with normal arteriography 9 years earlier. Neurochirurgie. 1989. 35: 15-22
21. Juhász J. Surgical treatment of arteriovenous angiomas localised in the corpus callosum, basal ganglia and near the brain stem. Acta Neurochir (Wien). 1978. 40: 83-101
22. Kemeny AA, Dias PS, Forster DM. Results of stereotactic radiosurgery of arteriovenous malformations: An analysis of 52 cases. J Neurol Neurosurg Psychiatry. 1989. 52: 554-8
23. Kohmura E, Taki T, Tanioka T. Multiple intracerebral arteriovenous malformations in deep structure – Case report. Neurol Med Chir (Tokyo). 1990. 30: 624-7
24. Kosary IZ, Braham J, Shacked I, Kronenberg Y. Vascular malformation of the posterior corpus callosum: Surgical treatment. Surg Neurol. 1978. 10: 345-7
25. Koyanagi I, Abe H, Nakagawa Y, Miyamachi K, Sasaki H, Miyasaka K. Nidus embolization of cerebral arteriovenous malformation fed mainly by a pericallosal artery prior to surgical excision. Case report. No Shinkei Geka. 1985. 13: 1019-24
26. Kunc Z. Arteriovenous malformations in the corpus callosum. Cesk Neurol Neurochir. 1974. 37: 253-7
27. Lobato RD, Gómez PA, Lagares A, Campollo J, González P, Boto GR. Parasplenial arteriovenous malformations. Report of 15 surgically treated cases. Neurocirugia (Astur). 2002. 13: 15-21
28. Machado de Almeida G, Shibata MK, Nakagawa EJ. Contralateral parafalcine approach for parasagittal and callosal arteriovenous malformations. Neurosurgery. 1984. 14: 744-6
29. Maruyama K, Shin M, Tago M, Kurita H, Kawamoto S, Morita A. Gamma knife surgery for arteriovenous malformations involving the corpus callosum. J Neurosurg. 2005. 102: S49-52
30. McDonald CR, Crosson B, Valenstein E, Bowers D. Verbal encoding deficits in a patient with a left retrosplenial lesion. Neurocase. 2001. 7: 407-17
31. Milhorat TH. Excision of a cirsoid arteriovenous malformation of the corpus callosum in a 16-year-old boy. Case report. J Neurosurg. 1970. 33: 339-44
32. Mohanty CB, Devi BI, Somanna S, Bhat DI, Dawn R. Corpus callosum arteriovenous malformation with persistent trigeminal artery. Br J Neurosurg. 2011. 25: 736-40
33. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010. 8: 336-41
34. Orozco LD, Luzardo GD, Buciuc RF. Transarterial balloon assisted onyx embolization of pericallosal arteriovenous malformations. J Neurointerv Surg. 2013. 5: e18-
35. Pan DH, Guo WY, Chung WY, Shiau CY, Chang YC, Wang LW. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000. 93: 113-9
36. Pendl G, Unger F, Papaefthymiou G, Eustacchio S. Staged radiosurgical treatment for large benign cerebral lesions. J Neurosurg. 2000. 93: 107-12
37. Picard L, Miyachi S, Braun M, Bracard S, Per A, Marchal JC. Arteriovenous malformations of the corpus callosum – Radioanatomic study and effectiveness of intranidus embolization. Neurol Med Chir (Tokyo). 1996. 36: 851-9
38. Pollock BE. Development and testing of a radiosurgery-based arteriovenous malformation grading system. Prog Neurol Surg. 2013. 27: 58-66
39. Robert T, Blanc R, Ciccio G, Gilboa B, Fahed R, Redjem H. Angiographic factors influencing the success of endovascular treatment of arteriovenous malformations involving the corpus callosum. J Neurointerv Surg. 2015. 7: 715-20
40. Sell SC. The corpus callosum, its role in memory: A presentation of a patient with an arteriovenous malformation of the corpus callosum. J Neurosurg Nurs. 1977. 9: 141-3
41. Shi YQ, Chen XC. Arteriovenous malformation of corpus callosum. Results of surgical treatment in 5 cases. Chin Med J (Engl). 1987. 100: 87-91
42. Shimizu S, Irikura K, Miyasaka Y, Mochizuki T, Kurata A, Kan S. Rupture of pial arteriovenous malformation associated with early thrombosis of the draining system following stereotactic radiosurgery – Case report. Neurol Med Chir (Tokyo). 2001. 41: 599-602
43. Starke RM, Yen CP, Ding D, Sheehan JP. A practical grading scale for predicting outcome after radiosurgery for arteriovenous malformations: Analysis of 1012 treated patients. J Neurosurg. 2013. 119: 981-7
44. Stefani MA, Porter PJ, terBrugge KG, Montanera W, Willinsky RA, Wallace MC. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke. 2002. 33: 920-4
45. Stefani MA, Porter PJ, terBrugge KG, Montanera W, Willinsky RA, Wallace MC. Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke. 2002. 33: 1220-4
46. Steiner L, Leksell L, Greitz T, Forster DM, Backlund EO. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972. 138: 459-64
47. Uchino A, Matsunaga M, Ohno M. Arteriovenous malformation of the corpus callosum associated with persistent primitive trigeminal artery – Case report. Neurol Med Chir (Tokyo). 1989. 29: 429-32
48. Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987. 110: 1631-46
49. Viñuela F, Dion JE, Duckwiler G, Martin NA, Lylyk P, Fox A. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: Experience with 101 cases. J Neurosurg. 1991. 75: 856-64
50. Wang YC, Lee SD, Chen NF, Shen CC. Cerebral intraventricular hemorrhage caused by a large cerebral arteriovenous malformation at 31 years after diagnosis. Zhonghua Yi Xue Za Zhi (Taipei). 2001. 64: 121-8
51. Yamamoto M, Hara M, Ide M, Ono Y, Jimbo M, Saito I. Radiation-related adverse effects observed on neuro-imaging several years after radiosurgery for cerebral arteriovenous malformations. Surg Neurol. 1998. 49: 385-97
52. Yasargil MG.editors. Microneurosurgery: AVM of the Brain, History, Embryology, Pathological Considerations, Hemodynamics, Diagnostic Studies, Microsurgical Anatomy. New York: Georg Thieme Verlag, Thieme Medical Publishers, Inc; 1987. p.
53. Yasargil MG, Jain KK, Antic J, Laciga R. Arteriovenous malformations of the splenium of the corpus callosum: Microsurgical treatment. Surg Neurol. 1976. 5: 5-14
54. Yasargil MG, Jain KK, Antic J, Laciga R, Kletter G. Arteriovenous malformations of the anterior and the middle portions of the corpus callosum: Microsurgical treatment. Surg Neurol. 1976. 5: 67-80
55. Zabel-du Bois A, Milker-Zabel S, Huber P, Schlegel W, Debus J. Stereotactic linac-based radiosurgery in the treatment of cerebral arteriovenous malformations located deep, involving corpus callosum, motor cortex, or brainstem. Int J Radiat Oncol Biol Phys. 2006. 64: 1044-8