- Department of Anaesthesiology, Critical Care and Pain, Tata Memorial Centre, Mumbai, India
- Department of Anaesthesia and Intensive Care, Division of Neuroanesthesia, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Anaesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India
- Department of Anaesthesiology and Critical Care, Armed Forces Medical College (AFMC), Pune, India
- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Nidhi Bidyut Panda, Professor Neuroanaesthesia, Division of Neuroanaesthesia, Department of Anaesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_25_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ketan Kataria1, Nidhi Bidyut Panda2, Ankur Luthra2, Shalvi Mahajan3, Hemant Bhagat2, Rajeev Chauhan3, Shiv Soni3, Kiran Jangra2, Narender Kaloria3, Shamik Paul4, Summit Bloria2, Shailesh Gupta2, Rajesh Chhabra5. Assessment of impaired cerebral autoregulation and its correlation with neurological outcome in aneurysmal subarachnoid hemorrhage: A prospective and observational study. 18-Aug-2023;14:290
How to cite this URL: Ketan Kataria1, Nidhi Bidyut Panda2, Ankur Luthra2, Shalvi Mahajan3, Hemant Bhagat2, Rajeev Chauhan3, Shiv Soni3, Kiran Jangra2, Narender Kaloria3, Shamik Paul4, Summit Bloria2, Shailesh Gupta2, Rajesh Chhabra5. Assessment of impaired cerebral autoregulation and its correlation with neurological outcome in aneurysmal subarachnoid hemorrhage: A prospective and observational study. 18-Aug-2023;14:290. Available from: https://surgicalneurologyint.com/surgicalint-articles/12508/
Abstract
Background: Cerebral autoregulation (CA) is crucial for the maintenance of cerebral homeostasis. It can be assessed by measuring transient hyperemic response ratio (THRR) using transcranial Doppler (TCD). We aimed at assessing the incidence of impaired CA (ICA) and its correlation with the neurological outcome in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Methods: One hundred consecutive patients with aSAH scheduled for aneurysmal clipping were enrolled in this prospective and observational study. Preoperative and consecutive 5-day postoperative THRR measurements were taken. Primary objective of the study was to detect the incidence of ICA and its correlation with vasospasm (VS) postclipping, and neurological outcome at discharge and 1, 3, and 12 months was secondary objectives.
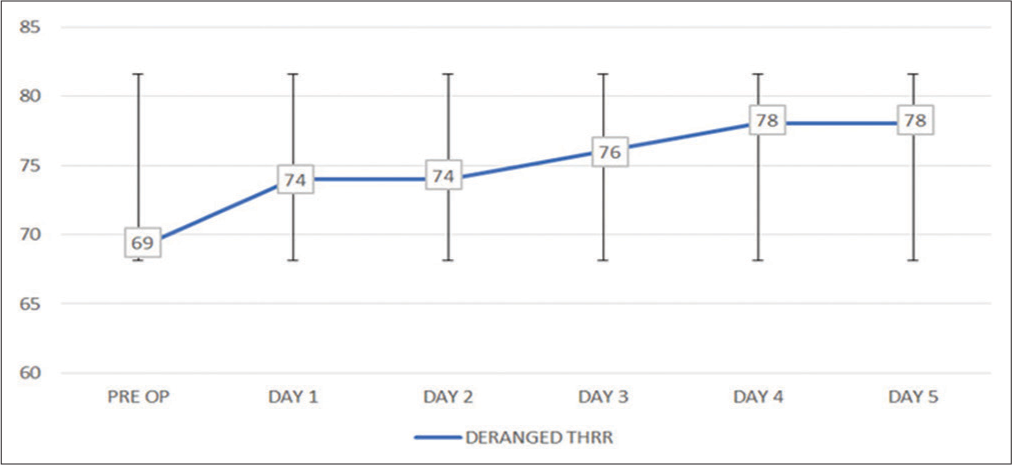
Results: ICA (THRR st and 2nd postoperative day, 76 patients on 3rd postoperative day, and 78 patients on 4th and 5th postoperative day. Significant VS was seen in 13.4% and 61.5% of patients with intact THRR and deranged THRR, respectively (P
Conclusion: Incidence of ICA assessed in aSAH patients varies from 69% to 78% in the perioperative period. The deranged CA was associated with significantly poor neurological outcome. Therefore, CA assessment using TCD-based THRR provides a simple, noninvasive bedside approach for predicting neurological outcome in aSAH.
Keywords: Cerebral autoregulation, Subarachnoid hemorrhage, Transcranial Doppler, Transient hyperemic response ratio
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is a neurosurgical emergency associated with a high rate of morbidity and mortality.[
Cerebral autoregulation (CA) has been defined as the intrinsic ability of the cerebral vasculature to maintain a constant cerebral blood flow over a wide range of mean arterial blood pressure (MAP) (50–150 mmHg).[
CA has two components – static and dynamic.[
We hypothesized that impaired CA (ICA) during initial period following aSAH can compromise cerebral perfusion which leads to poor neurological outcome even after successful surgical obliteration of aneurysm. Due to a dearth of published data on the occurrence of ICA in aSAH, we designed this prospective and observational study to determine the incidence of ICA in different grades of aSAH and to correlate ICA with neurological prognosis. The primary aim of the study was to detect the incidence of ICA by measurement of transient hyperemic response ratio (THRR) using TCD. Correlation of ICA with vasospasm (VS) postclipping and neurological outcome as measured by modified Rankin Scale (mRS) at discharge and Glasgow outcome scale extended (GOSE) at 1, 3, and 12 months was secondary outcomes of the study.
MATERIALS AND METHODS
A prospective and observational study was conducted in patients with aSAH admitted to a high-volume referral tertiary care institute. After obtaining the Institutes Ethics Committee approval (NK/3971/DM) and clinical trial registry India registration (CTRI/2018/02/011932), 100 consecutive aSAH patients planned for surgical clipping of aneurysm were enrolled in the study.
Patients aged >18 years and <65 years, having all grades of aneurysms according to Hunt and Hess (H&H), World Federation of Neurosurgical Society (WFNS), and Modified Fisher grades were included in the study. H&H grades (IV and V), WFNS grade (IV and V), and modified Fisher grade (III and IV) were considered as poor grade aSAH. Patients with carotid artery disease, ischemic stroke, hemodynamic instability, inadequate TCD window, angiographic VS on initial computed tomography angiography/digital subtraction angiography (DSA), and ruptured posterior circulation aneurysms were not included in the study. Patient or patient’s next kin, if refuse to participate in the study, was also excluded from the study.
All patients underwent a detailed preanesthetic evaluation before surgery. A written informed consent was obtained from the patient or patient’s next kin.
TCD protocol
In the preoperative period, bilateral carotid Doppler was performed to rule out carotid plaque or atheroma before enrolling the patients in the study to avoid the disruption and dislodgement of the plaque during THRR test. Elderly patients (defined by the World Health Organization as those being above 65 years of age) who have a higher prevalence of arterial atheromatous plaques were excluded from the study. In addition, the manipulation of the carotid in the neck appears to be an ostensibly harmless procedure, but it can occasionally lead to significant problems such as hypotension and bradycardia.[
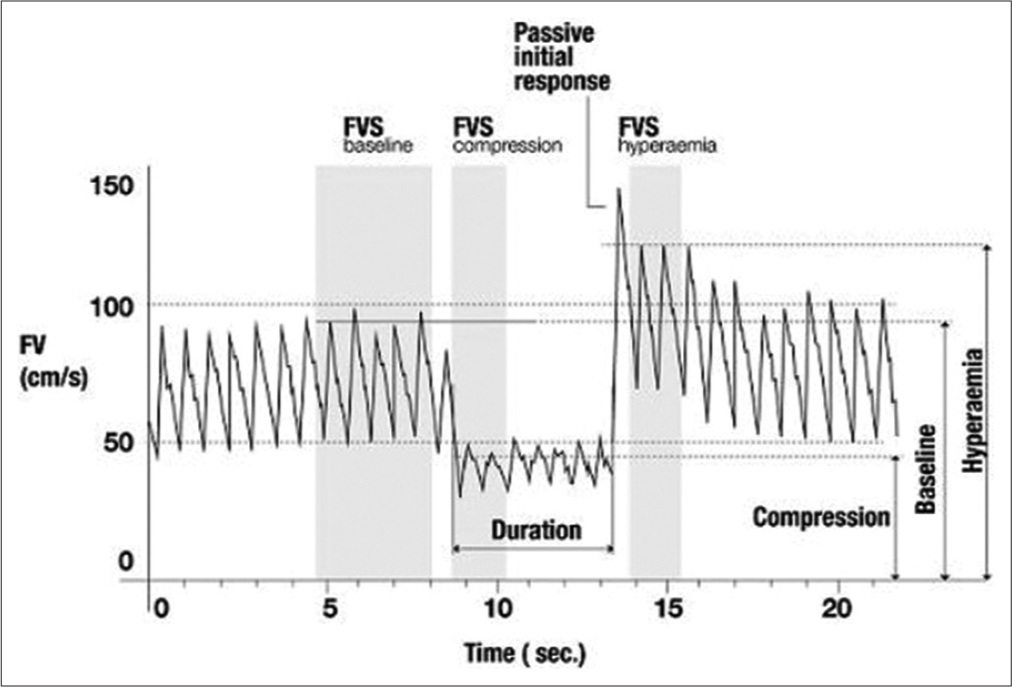
The TCD measurements were performed keeping patient in supine position. A 2 MHz frequency Doppler probe was used to insonate middle cerebral artery (MCA) at a depth of 30–60 mm, through temporal window using a TCD machine (RIMED Healthcare, Israel). The angle between the probe and skull was readjusted to obtain an optimal signal. The peak systolic flow velocity (FVs), end diastolic FV, and mean FV were assessed as cerebral blood flow velocities.
For measurement of THRR, the test was performed using Giller’s maneuver with TCD probe in place on the side of the ruptured aneurysm while manually compressing the ipsilateral common carotid artery, with compression ratio of more than 50% of the baseline for 10 s.[
The result of the tests measured for THRR was accepted if following criteria were met: (a) there was maximum and sudden decrease in MCA FV after compressing the carotid artery with no further decline in MCA FV, (b) heart rate and mean blood pressure remained stable during compression (i.e., within 10% of their respective precompression values), and (c) the reflected Doppler signal’s power remained the same during the test.
Anesthesia protocol
In the intraoperative and postoperative period, a standard anesthesia technique and standard intensive care protocol, respectively, were followed in all the cases according to the institutional protocol. All surgeries were performed by a single experienced neurosurgeon in the early post-SAH phase (within first 3 days of arrival to the hospital post ictus). In the postoperative period, THRR measurements by TCD were performed in the patients for consecutive 5 postoperative days.
Incidence of cerebral VS was noted as per clinical, TCD, and angiographic criteria. Clinical criteria for cerebral VS were new onset neurological deterioration (hemiparesis, apraxia, neglect, aphasia, and hemianopia) or fall in Glasgow Coma Scale by >2 points and the duration of symptoms lasted for at least 1 h. An alert was sound, if there was increased cerebral blood FV on routine daily TCD evaluation of such patients. TCD confirmation of VS was done when mean cerebral blood FV in MCA was >120 cm/s and Lindegaard ratio was >3. Emergency angiographic study was performed within an hour of neurological deterioration.
At the time of discharge, mRS score was assessed and more than three score (mRS >3) was considered as unfavorable outcome. Later, all patients were followed up telephonically at 1, 3, and 12 months after discharge. Neurological outcome was evaluated using GOSE. Less than seven GOSE score was considered as unfavorable outcome. Correlation of ICA with neurological outcome as measured by mRS at discharge and GOSE at 1, 3, and 12 months was analyzed.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp). Normality of quantitative data was checked by Kolmogorov–Smirnov tests of normality. The continuous data were presented as mean ± standard deviation (SD) or median and inter quartile range, as appropriate. All measurable normally distributed data were expressed as mean and SD. Categorical and classified data were compared by χ2 test or Fisher’s exact test. Receiver operating characteristic (ROC) curve was drawn between ICA and neurological outcome to assess the sensitivity and specificity of THRR test with neurological outcome. P < 0.05 was regarded significant.
RESULTS
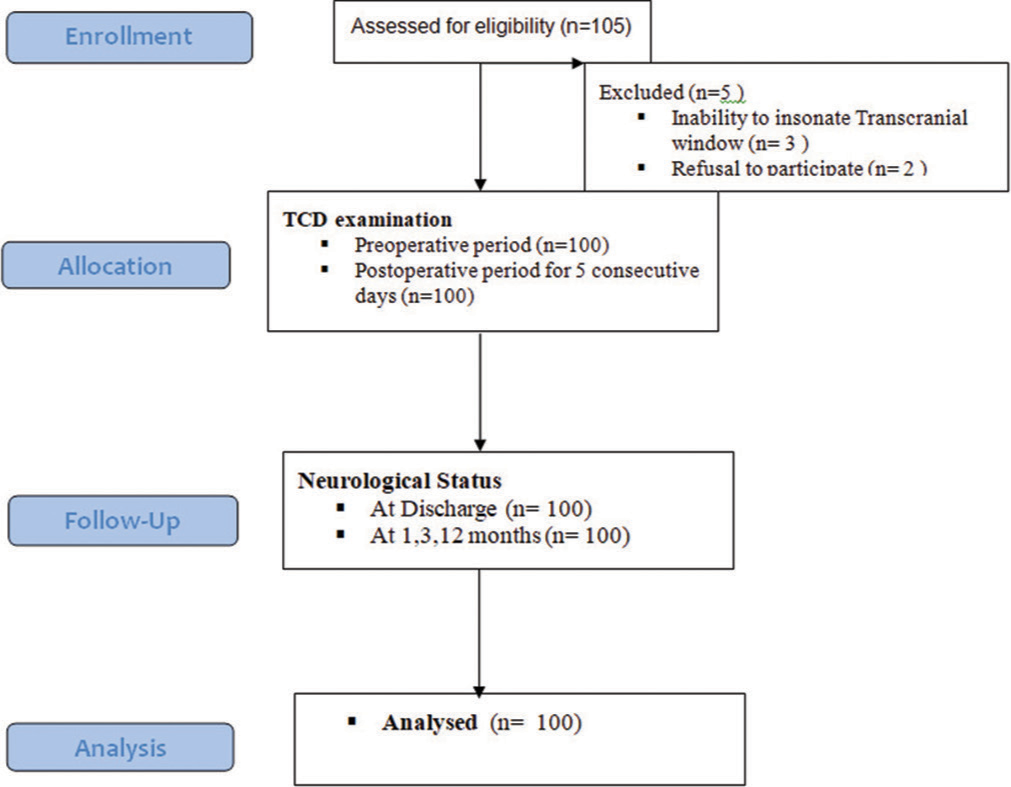
The study assessed a total of 105 patients, of whom five were excluded due to the inability to perform TCD insonation in three cases and the patient’s family refusing to participate in two cases. As a result, 100 patients were included in the study and their data were analyzed [
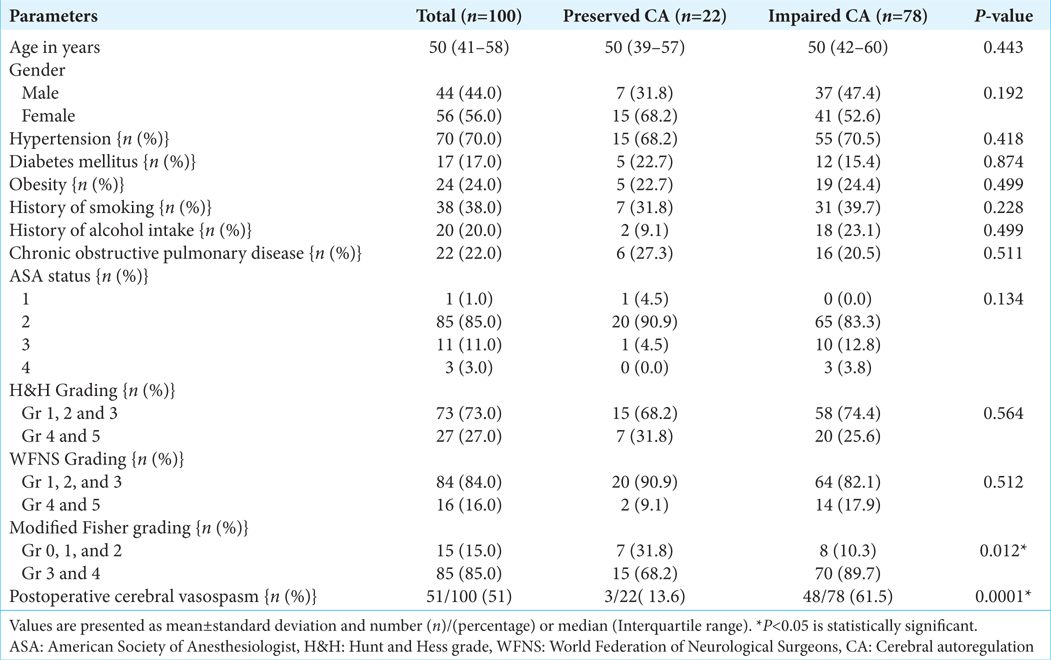
All of the patients who participated in the study were separated into two groups: those who had preserved CA and those who had ICA. Demographic parameters and other baseline characteristics were comparable between these two groups [
Association of grading of aneurysms (H&H grading, WFNS grading, and Modified fisher grading) with the status of CA of the patients is shown in
During postoperative period, 51% of patients had angiographically proven cerebral VS. Significant cerebral vasospasm was present in 3 patients (13.4%) out of 22 patients with intact THRR and 48 (61.5%) patients out of 78 patients with deranged THRR.
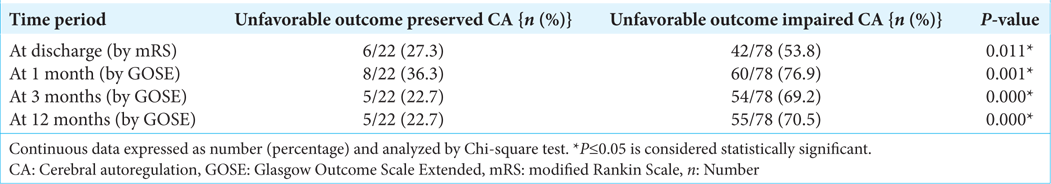
Among 78 patients of ICA, 42 (53.8%) patients had unfavorable outcomes at discharge (mRS ≥ 3). The difference between unfavorable and favorable outcomes in patients with ICA was statistically significant with P = 0.020 [
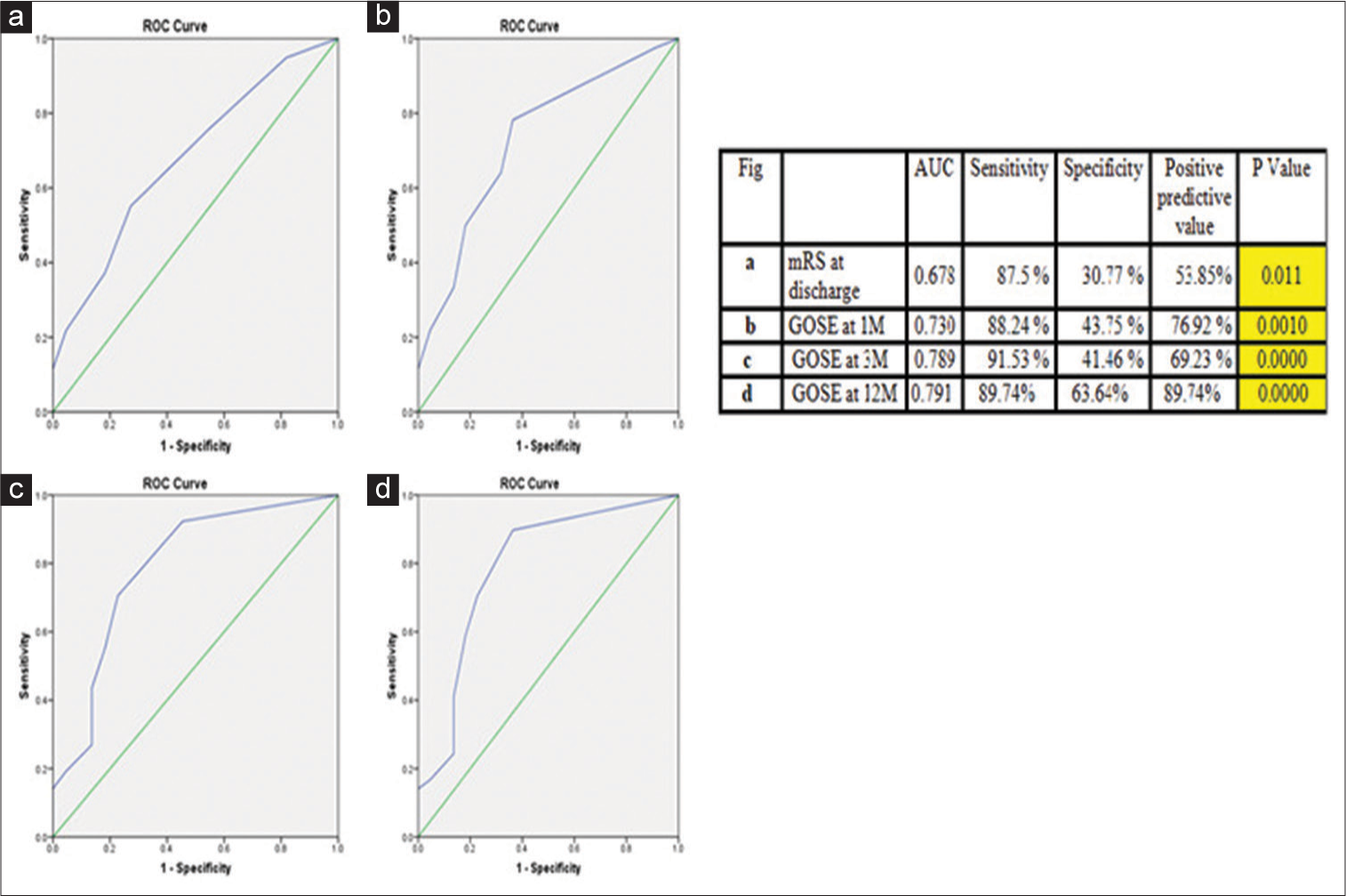
Figure 4:
Receiver operating characteristic (ROC) curves analysis for unfavorable outcome in patients with impaired cerebral autoregulation (deranged transient hyperemic response ratio). (a) ROC at discharge, (b) ROC 1 month, (c) ROC at 3 months, and (d) ROC at 12 months. In Figure 4 the Green line is the Reference line (AUC=0.5) and the Blue line indicates ROC curve. Impaired Cerebral Autoregulation (assessed by deranged Transient Hyperemic Response Ratio) was associated with an unfavorable outcome. Area under curve (AUC) increased from discharge (Fig a) to maximum at follow up at 12 months (Fig d). Thus, the ROC curve shows that deranged transient hyperemic response ratio predicts unfavorable outcome with high sensitivity and specificity. Yellow indicates highly statistically significant. AUC: Area under the curve, mRS: modified rankin scale, GOSE: Glasgow outcome scale extended.
During post discharge follow-up at 1 month, it was noted that the unfavorable outcome (GOSE < 7) was seen in 60 (76.9%) patients among ICA [
During post discharge follow-up at 3 months by GOSE showed that out of 78 patients of ICA, 54 (69.2%) patients had unfavorable outcome (GOSE ≤ 7) [
In 12 months post discharge follow-up, we found out of 78 patients with THRR <1.09, 55 (70.5%) patients had unfavorable outcome [
DISCUSSION
This prospective and observational study explored the incidence of deranged CA using THRR, in 100 consecutive aSAH patients who underwent surgical clipping following rupture of anterior cerebral circulation aneurysm. It was observed that 69% aSAH patients had ICA in post ictus (preoperative) period which increased to 78% on the postoperative day 5. Out of 78 patients, who had ICA, 53.8% (n = 42) at discharge, 76.9% (n = 60) at 1 month, 69.2% (n = 54) at 3 months, and 70.5% (n = 55) patients at 12 months had unfavorable neurological outcome, which was significantly higher than those with preserved CA.
CA is the elemental competency of cerebral arterial vessels to regulate their diameter, to uphold a proportionately consistent cerebral blood flow with shifts in cerebral perfusion pressure. Disturbance of CA over the early course of aSAH, conceivably increases the risk of delayed cerebral ischemia (DCI) and adversely impacts the long-term functional outcomes.[
The carotid compression test, also referred to as the THR test, was initially proposed by Giller as a clinical assessment tool for CA.[
TCD can evaluate the status of CA by measuring the changes in peak flow velocity (PFV) in intracerebral arteries by briefly compressing the ipsilateral common carotid artery. According to Smielewski et al. well cited work, “the usual values of THRR measured in healthy individuals were between 1.105 and 1.29.”[
Disturbance of CA over the early course of aneurysmal SAH is increasingly known to cause VS, cerebral infarction, and poor neurological outcome.[
In the present study, all the patients were followed up at discharge using modified Rankin Score and at 1, 3, and 12 months after discharge using GOSE. We found a significant correlation between ICA and unfavorable GOSE at 1, 3, and 12 months. (P = 0.001, <0.001 and <0.001 respectively). Hence, deranged CA correlated well with unfavorable outcomes. Rynkowski et al. mentioned that patients in ICA group advanced to an unfavorable outcome compared to the preserved CA group (73.7% vs. 4.7%, P = 0.0001).[
Fontana et al. studied the course of dynamic autoregulation by leg cuff test and dynamic ARI for initial 8 days following SAH and its association with angiographic VS and clinical outcome measured by mRS in 51 SAH patients prospectively.[
ICA may have a key role to play in the development of VS, DCI, cerebral infarction which in turn may result in poor neurological outcomes. Numerous pathological processes other than large vessel narrowing have been hypothesized. These include brain injury caused by global cerebral ischemia as a result of increased intracranial pressure secondary to initial bleeding, cortical spreading ischemia, microcirculatory VS, and cerebral micro thrombosis. However, it is not clear if these processes are discrete expressions of the same cerebrovascular failure or if they work as distinct entities.
Limitations
There were few limitations in our study. The study was limited to those patients who underwent clipping surgery. Although this gave homogeneity to the study population, patients undergoing coiling or managed conservatively could not be evaluated in the study. Postoperative rehabilitation programs and cognitive therapy which have vital influence on neurological outcome were not taken into account in the study while assessing postdischarge follow-up of the patient. A multi-centric study would have provided a more reliable validation of our findings with a larger sample size.
CONCLUSION
THRR measured by Transcranial Doppler is a simple, bedside, noninvasive, and repeatable test for assessment of CA. The incidence of ICA in patients with aneurysmal SAH evaluated by THRR using TCD varied from 69% to 78% in the perioperative period. The impaired autoregulation may predict unfavorable outcomes at discharge and poor neurological outcome at 1, 3, and 12 months. Further studies are needed to use THRR in different groups of patients with acute cerebral insult to focus on early recognition of ICA and target intensive therapies to improve overall neurological outcome.
List of drugs used
These standard drugs were used for induction and maintenance of anesthesia during surgery
1) Propofol 2) Fentanyl 3) Vecuronium 4) Phenytoin.
Ethics approval and consent to participate
Clearance to conduct the study was obtained from the Institute Ethics Committee in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from either patients or next of the kin all patients who participated in the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989. 20: 45-52
2. Al-Jehani H, Angle M, Marcoux J, Teitelbaum J. Early abnormal transient hyperemic response test can predict delayed ischemic neurologic deficit in subarachnoid hemorrhage. Crit Ultrasound J. 2018. 10: 1
3. Budohoski KP, Czosnyka M, Kirkpatrick PJ, Reinhard M, Varsos GV, Kasprowicz M. Bilateral failure of cerebral autoregulation is related to unfavorable outcome after subarachnoid hemorrhage. Neurocrit Care. 2015. 22: 65-73
4. Calverley JR, Millikan CH. Complications of carotid manipulation. Neurology. 1961. 11: 185-9
5. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2012. 43: 1711-37
6. Czosnyka M, Miller C. Participants in the International Multidisciplinary Consensus Conference on multimodality monitoring: Monitoring of cerebral autoregulation. Neurocrit Care. 2014. 21: S95-102
7. Dernbach PD, Little JR, Jones SC, Ebrahim ZY. Altered cerebral autoregulation and CO2 reactivity after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1988. 22: 822-6
8. Fontana J, Moratin J, Ehrlich G, Scharf J, Weiß C, Schmieder K. Dynamic autoregulatory response after aneurysmal subarachnoid hemorrhage and its relation to angiographic vasospasm and clinical outcome. Neurocrit Care. 2015. 23: 355-63
9. Fontana J, Wenz H, Schmieder K, Barth M. Impairment of dynamic pressure autoregulation precedes clinical deterioration after aneurysmal subarachnoid hemorrhage. J Neuroimaging. 2016. 26: 339-45
10. Gaasch M, Schiefecker AJ, Kofler M, Beer R, Rass V, Pfausler B. Cerebral autoregulation in the prediction of delayed cerebral ischemia and clinical outcome in poor-grade aneurysmal subarachnoid hemorrhage patients. Crit Care Med. 2018. 46: 774-80
11. Giller CA. A bedside test for cerebral autoregulation using transcranial Doppler ultrasound. Acta Neurochir (Wien). 1991. 108: 7-14
12. Hop JW, Rinkel GJ, Algra A, van Gijn J. Case fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke. 1997. 28: 660-4
13. Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007. 38: 981-6
14. Lam JM, Smielewski P, Czosnyka M, Pickard JD, Kirkpatrick PJ. Predicting delayed ischemic deficits after aneurysmal subarachnoid hemorrhage using a transient hyperemic response test of cerebral autoregulation. Neurosurgery. 2000. 47: 819-25
15. Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: The phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001. 29: 158-63
16. Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959. 39: 183-238
17. Lindegaard KF. The role of transcranial Doppler in the management of patients with subarachnoid haemorrhage--a review. Acta Neurochir Suppl (Wien). 1999. 72: 59-71
18. Moerman AT, Vanbiervliet VM, Van Wesemael A, Bouchez SM, Wouters PF, De Hert SG. Assessment of cerebral autoregulation patterns with near-infrared spectroscopy during pharmacological-induced pressure changes. Anesthesiology. 2015. 123: 327-35
19. Rätsep T, Asser T. Cerebral hemodynamic impairment after aneurysmal subarachnoid Hemorrhage as evaluated Using transcranial Doppler ultrasonography: Relationship to delayed cerebral ischemia and clinical outcome. J Neurosurgery. 2001. 95: 393-401
20. Rätsep T, Eelmäe J, Asser T. Routine utilization of the transient hyperaemic response test after aneurysmal subarachnoid haemorrhage. Acta Neurochir Suppl. 2002. 81: 121-4
21. Rynkowski CB, de Oliveira Manoel AL, Dos Reis MM, Puppo C, Worm PV, Zambonin D. Early transcranial doppler evaluation of cerebral autoregulation independently predicts functional outcome after aneurysmal subarachnoid haemorrhage. Neurocrit Care. 2019. 31: 253-62
22. Smielewski P, Czosnyka M, Iyer V, Piechnik S, Whitehouse H, Pickard J. Computerised transient hyperaemic response test--a method for the assessment of cerebral autoregulation. Ultrasound Med Biol. 1995. 21: 599-611
23. Smielewski P, Czosnyka M, Kirkpatrick P, Pickard JD. Evaluation of the transient hyperemic response test in head-injured patients. J Neurosurg. 1997. 86: 773-8
24. Soehle M, Czosnyka M, Pickard JD, Kirkpatrick PJ. Continuous assessment of cerebral autoregulation in subarachnoid hemorrhage. Anesth Analg. 2004. 98: 1133-9
25. Svedung WT, Howells T, Lewén A, Ronne EE, Enblad P. Temporal dynamics of ICP, CPP, PRx, and CPP opt in high-grade aneurysmal subarachnoid hemorrhage and the relation to clinical outcome. Neurocrit Care. 2021. 34: 390-402
26. Sviri GE, Ghodke B, Britz GW, Douville CM, Haynor DR, Mesiwala AH. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006. 59: 360-6
27. Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995. 26: 1014-9
28. Van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: An overview of current concepts, and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008. 28: 1071-85