- Department of Neurosurgery, Yamagata University, Yamagata, Japan
- Department of Biomedical Research and Innovation, National Hospital Organization Osaka National Hospital, Osaka, Japan
- Department of Radiology, Division of Diagnostic Radiology, Yamagata University, Yamagata, Japan.
- Department of Pathological Diagnostics, Yamagata University, Yamagata, Japan.
Correspondence Address:
Yuta Mitobe, Department of Neurosurgery, Yamagata University, Yamagata, Japan.
DOI:10.25259/SNI_63_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shigeru Kamimura1, Yuta Mitobe1, Kazuki Nakamura1, Kenichiro Matsuda1, Yonehiro Kanemura2, Masafumi Kanoto3, Mitsuru Futakuchi4, Yukihiko Sonoda1. Association of ADC of hyperintense lesions on FLAIR images with TERT promoter mutation status in glioblastoma IDH wild type. 29-Mar-2024;15:108
How to cite this URL: Shigeru Kamimura1, Yuta Mitobe1, Kazuki Nakamura1, Kenichiro Matsuda1, Yonehiro Kanemura2, Masafumi Kanoto3, Mitsuru Futakuchi4, Yukihiko Sonoda1. Association of ADC of hyperintense lesions on FLAIR images with TERT promoter mutation status in glioblastoma IDH wild type. 29-Mar-2024;15:108. Available from: https://surgicalneurologyint.com/surgicalint-articles/12831/
Abstract
Background: Although mutations in telomerase reverse transcriptase (TERT) promoter (TERTp) are the most common alterations in glioblastoma (GBM), predicting TERTp mutation status by preoperative imaging is difficult. We determined whether tumour-surrounding hyperintense lesions on fluid-attenuated inversion recovery (FLAIR) were superior to those of contrast-enhanced lesions (CELs) in assessing TERTp mutation status using magnetic resonance imaging (MRI).
Methods: This retrospective study included 114 consecutive patients with primary isocitrate dehydrogenase (IDH)-wild-type GBM. The apparent diffusion coefficient (ADC) and volume of CELs and FLAIR hyperintense lesions (FHLs) were determined, and the correlation between MRI features and TERTp mutation status was analyzed. In a subset of cases, FHLs were histopathologically analyzed to determine the correlation between tumor cell density and ADC.
Results: TERTp mutations were present in 77 (67.5%) patients. The minimum ADC of FHLs was significantly lower in the TERTp-mutant group than in the TERTp-wild-type group (mean, 958.9 × 10−3 and 1092.1 × 10−3 mm2/s, respectively, P
Conclusion: The ADC of FHLs was significantly lower in IDH-wild-type GBM with TERTp mutations, suggesting that determining the ADC of FHLs on preoperative MRI might be helpful in predicting TERTp mutation status and surgical planning.
Keywords: Apparent diffusion coefficients (ADC), Fluid-attenuated inversion recovery hyperintense lesion, Glioblastoma, Telomerase reverse transcriptase (TERT)
INTRODUCTION
Glioblastoma (GBM) is the most common primary malignant tumor of the central nervous system in adults.[
Several genetic markers, such as methylguanine methyltransferase promoter unmethylation, phosphatase and tensin homolog gene mutations, and epidermal growth factor receptor gene amplification, are associated with poor survival in patients with GBM.[
Necrosis detected by MRI has been reported to indicate the presence of TERTp mutations.[
We recently reported that TERTp mutations were strongly associated with poor prognosis and multifocal/distant lesions in isocitrate dehydrogenase (IDH)-wild-type GBMs.[
Therefore, we aimed to determine if the TERTp mutation status correlated with MRI features, with a focus on the ADC of FHLs.
MATERIALS AND METHODS
Patients and samples
This retrospective study was conducted with the approval of the Ethics Committee of the Yamagata University Faculty of Medicine (Ethical Approval Number: 2021-214, Ethical Approval Date: 2021/9/3), and written informed consent using the opt-out approach was obtained from all patients or their families. Among 115 consecutive patients treated with surgical removal of the tumor followed by adjuvant chemoradiotherapy with irradiation and/or TMZ between January 2009 and October 2021, 114 patients who met the following inclusion criteria were included: (1) diagnosis of grade 4 IDH-wild-type GBM according to the 2021 World Health Organization classification;[
In all patients, tumor specimens were obtained from lesions that exhibited enhancement on gadolinium-enhanced MRI scans and immediately stored at −80°C until DNA extraction.
MRI
All patients underwent preoperative MRI within seven days before surgery with a 1.5- or 3.0-T Achieva MRI scanner (Philips Medical Systems, Amsterdam, Netherlands). For data collection and evaluation, the Picture Archiving and Communication System (Impax ee, Agfa Healthcare, Bonn, Germany) was used. Diffusion-weighted imaging was conducted with b-values of 0 and 1000 s/mm2 to generate ADC maps. Sequences were obtained using 30 slices with a slice thickness of 5 mm. In the FLAIR examination, the following settings were used: set repetition time, 9000 ms; echo time, 125 ms; inversion time, 2500 ms; and slice thickness, 6 mm. First, FLAIR images were used to set several regions of interest (ROIs) in almost all FHLs surrounding the CEL; the ROIs were placed at least 3 mm away from the CEL in each tumor in the FLAIR images, as we previously reported.[
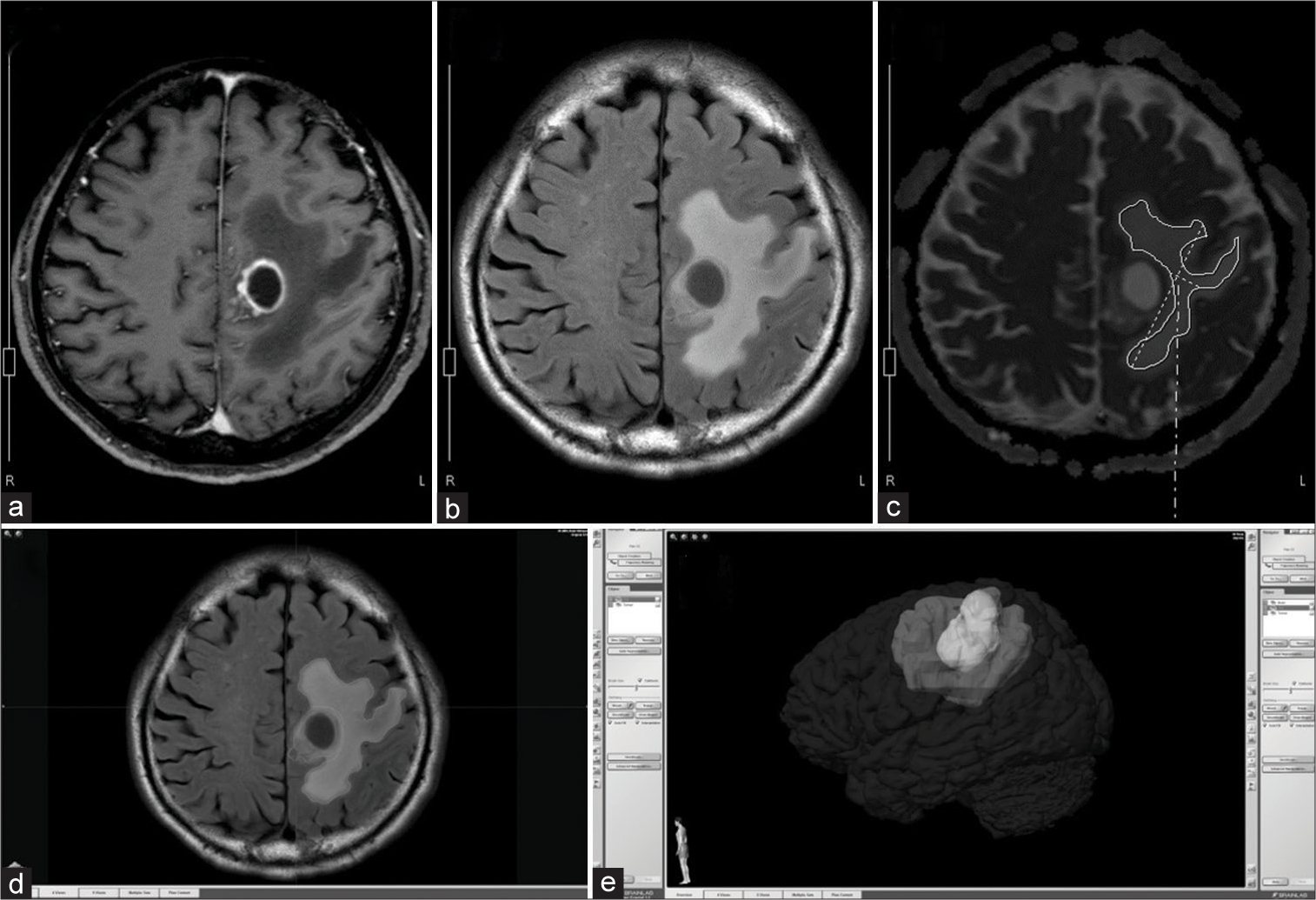
Figure 1:
Image analysis of preoperative magnetic resonance imaging scans. Several regions of interest (ROIs) of hyperintense lesions on fluid-attenuated inversion recovery (FLAIR) high lesion (FHLs) at least 3 mm away from (a) the contrast-enhanced lesion (CEL) on (b) FLAIR. (c) Next, apparent diffusion coefficients (ADCs) of corresponding ROIs in FHLs were calculated on the ADC map. CEL and FHL volumes were measured after image fusion of contrast-enhanced T1-weighted and FLAIR images. (d) Segmentations of CEL and FHL were based on differences in signal intensity using the semiautomatic “smartbrush” tool. (e) Finally, 3D volumetric evaluations of CELs and FHLs were performed. FHL: FLAIR high lesion.
Tumor and FHL volumes were measured after image fusion using iPlan 3.0 (BrainLab AG). On gadolinium-enhanced and FLAIR images, CEL and FHL segmentations based on differences in signal intensity were created using the “smart brush” tool. Next, volumetric evaluations of CELs and FHLs were completed using multiple ROIs [
Molecular analysis
Genomic DNA was extracted using the QIAamp DNA mini kit (Qiagen), according to the manufacturer’s instructions. Mutation status for IDH1, IDH2, and TERTp was analyzed using Sanger sequencing, as described previously.[
Statistical analysis
Statistical analyses were performed using SPSS ver. 26 (IBM Japan, Tokyo, Japan). Relationships between two variables were evaluated using the Mann–Whitney U-test. Pearson’s correlation coefficient analysis was used to evaluate the association between ADC and tumor cell density.
Histopathological analysis of FHLs
For the comparison of tumor cell density and ADC of biopsied FHLs, navigation-guided multiple tumor sampling of FHLs was performed in the last ten consecutive patients with GBM who had available tissue samples. Tumor cell counts were determined in three high-powered fields of view (400×) in each sample, as previously described.[
RESULTS
Patient characteristics
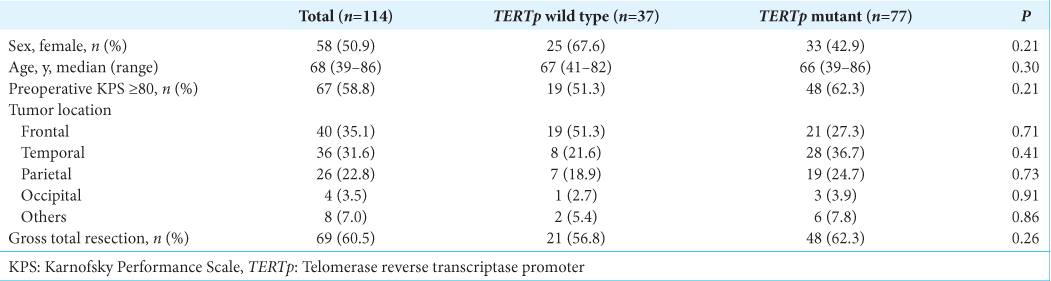
Among 115 cases with the histological features of GBM, one patient with IDH1 mutation was excluded from the study. Therefore, a total of 114 patients with IDH-wild type GBM, including 56 male and 58 female patients, with a median age of 68 years (range 39–86 years), were included in the present study. The tumors were located in the frontal, temporal, parietal, and occipital lobes in 40, 36, 26, and 4 patients, respectively, and in the insula, basal ganglia, and corpus callosum in 5, 2, and 1 patient, respectively. Gross total surgical resection was performed in 69 (60.5%) patients. TERTp mutations were found in 77 of the 114 (67.5%) patients; of these, 89 patients were previously reported.[
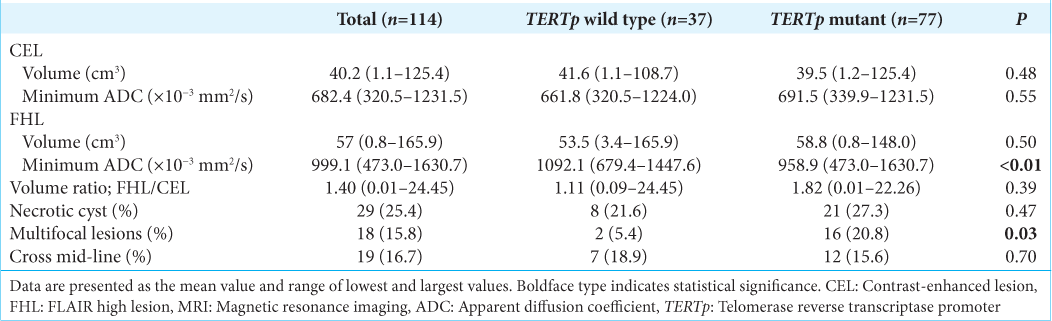
ADC volume and value of CELs and FHLs
As shown in
The minimum ADC of CELs was not significantly different between the TERTp-wild type and TERTp-mutant groups (mean, 661.8 × 10−3 and 691.5 × 10−3 mm2/s, respectively; P = 0.55) [
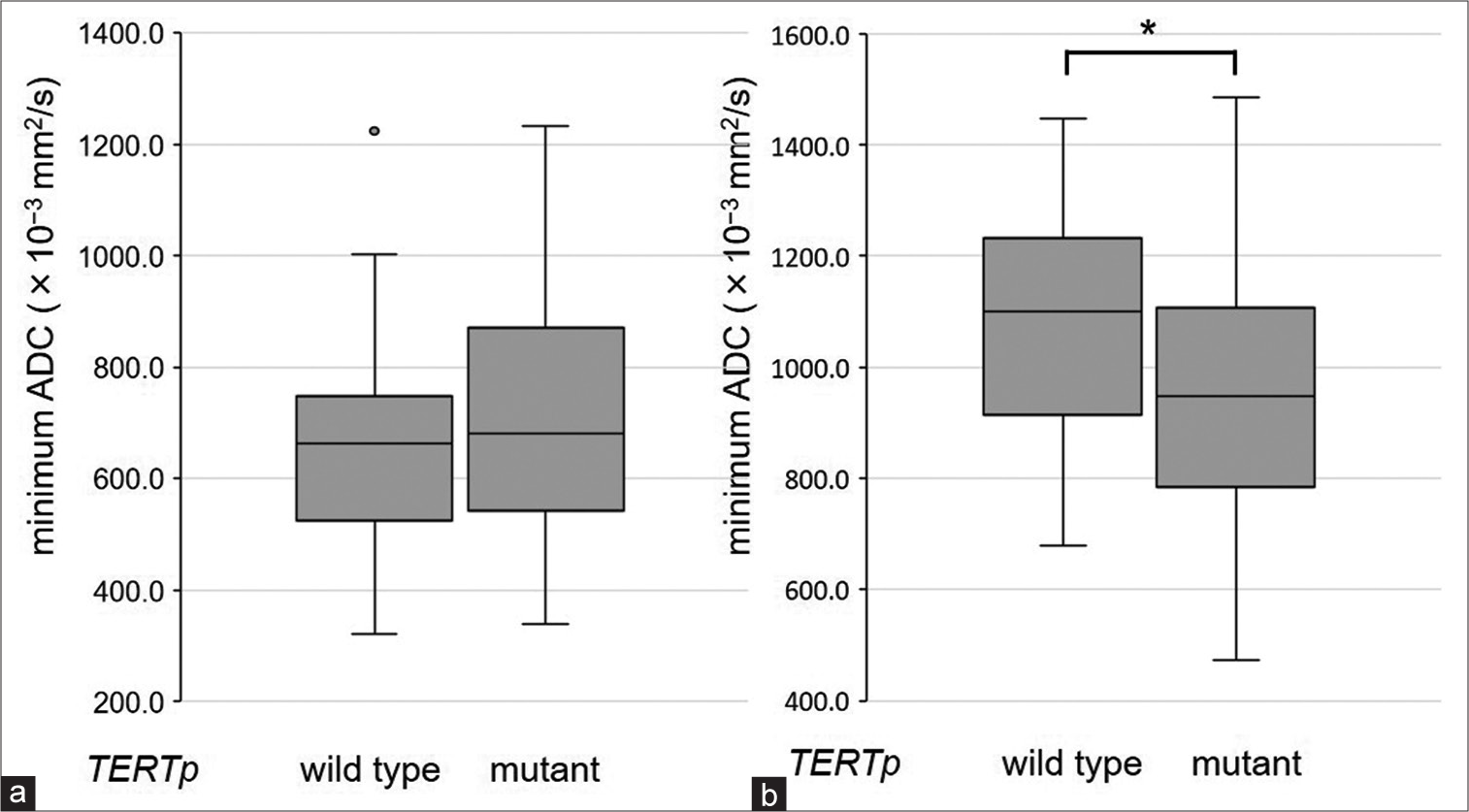
Figure 2:
Bar graphs show the comparison of minimum apparent diffusion coefficients values of (a) contrast-enhanced lesions and (b) FLAIR hyperintense lesions between the groups without or with telomerase reverse transcriptase promoter mutations. Relationships between the two groups were evaluated using the Mann–Whitney U-test (*P < 0.05).
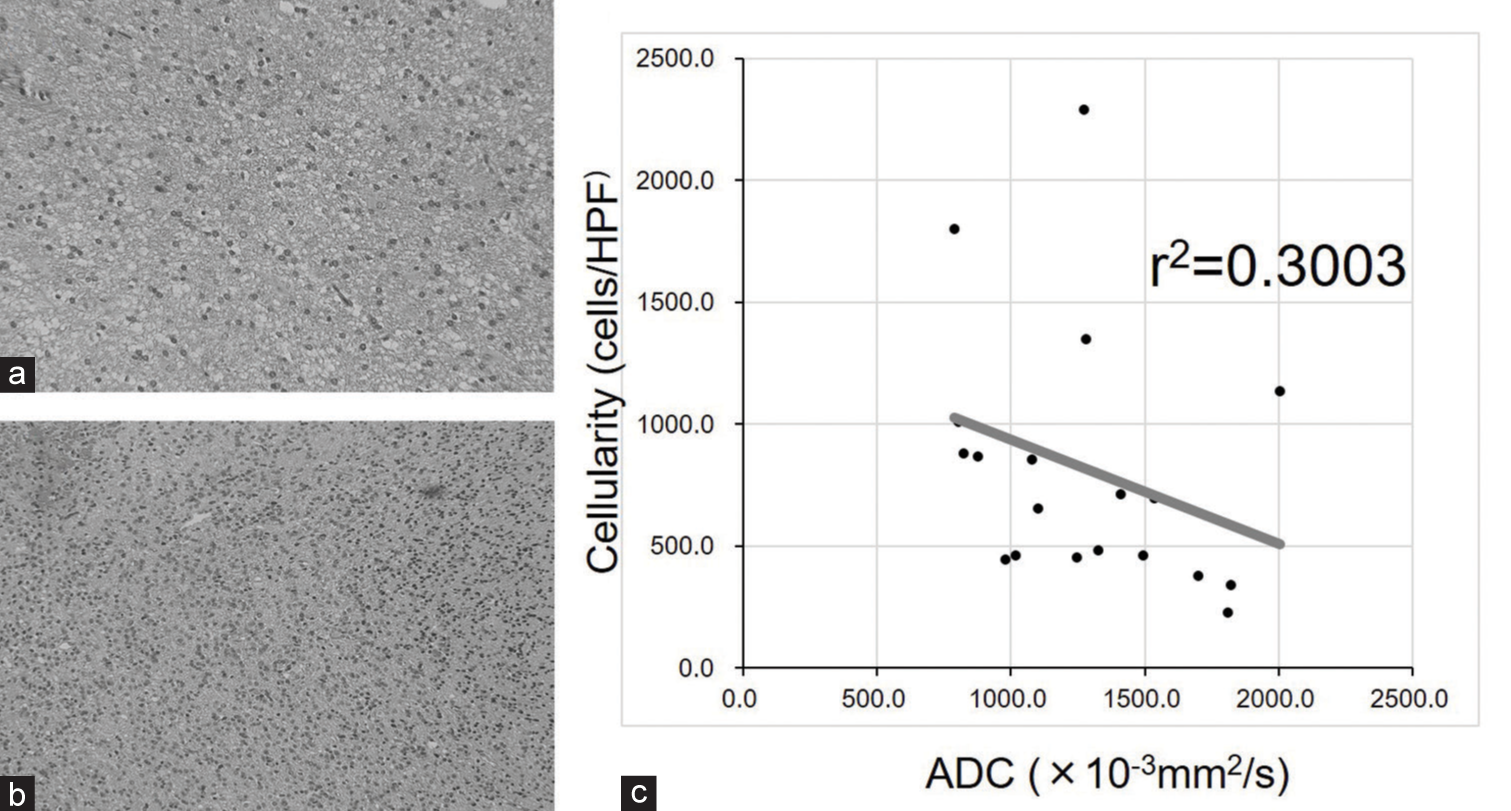
Correlation between the tumor cell density and ADC of FHLs
As shown in
Figure 3:
Histological features of FLAIR hyperintense lesions. (a) Note sparse tumor cells in a tissue sample collected from a lesion with high apparent diffusion coefficient (ADC) (1534.9 × 10−3 mm2/s). (b) Note high tumor cell density in a tissue sample collected from a lesion with low ADC (1272.5 × 10−3 mm2/s). (c) There was a trend toward a negative correlation between ADC and tumor cell density, although a statistically significant correlation could not be observed due to the small number of tumor samples.
DISCUSSION
TERTp mutations are the most common genetic abnormality in GBM. The previous studies suggested that TERTp mutations were involved in multiple lesions, remote recurrence, and poor prognosis in GBM.[
ADC, a well-known parameter associated with tumor cell density and infiltration, has been used to predict prognosis and evaluate recurrent lesions in patients with GBM.[
In the present study, we could not detect differences in the MRI features of CELs and FHLs between the TERTp-wild type and TERTp-mutant groups. These results confirm previous studies suggesting that predicting TERTp mutation status by preoperative MRI alone was difficult.[
In the present study, we also confirmed the findings of a previous study, which reported that the ADC of CELs did not differ between TERTp wild type and TERTp-mutant GBMs.[
Several recent studies reported the clinical utility of the additional removal of FHLs beyond gross total resection.[
This study has several limitations. First, this was a single-center study, including the analysis of retrospectively collected datasets. Second, there were relatively small cases in which tumor cell density was determined with histological evaluation.
CONCLUSION
ADC of FHLs was significantly lower in GBMs with TERTp mutations than in those with wild-type TERTp. These results suggest that the ADC of FHLs on preoperative MRI might be helpful in predicting the TERTp mutation status and surgical planning.
Ethical approval
The research/study approved by the Institutional Review Board at the Ethical Review Committee of Yamagata University Faculty of Medicine, number 2021–214, dated 2021/9/3.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
JSPS KAKENHI Grant Number JP19K18377 and JP20K09363.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Burth S, Kickingereder P, Eidel O, Tichy D, Bonekamp D, Weberling L. Clinical parameters outweigh diffusion-and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro Oncol. 2016. 18: 1673-9
2. Campos MA, Macedo S, Fernandes M, Pestana A, Pardal J, Batista R. TERT promoter mutations are associated with poor prognosis in cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2019. 80: 660-9.e6
3. Certo F, Altieri R, Maione M, Schonauer C, Sortino G, Fiumanò G. FLAIRectomy in supramarginal resection of glioblastoma correlates with clinical outcome and survival analysis: A prospective, single institution, case series. Oper Neurosurg (Hagerstown). 2021. 20: 151-63
4. Chang PD, Chow DS, Yang PH, Filippi CG, Lingnelli A. Predicting glioblastoma recurrence by early changes in the apparent diffusion coefficient value and signal intensity on FLAIR images. Am J Radiol. 2017. 208: 57-65
5. Chang W, Pope WB, Harris RJ, Hardy AJ, Leu K, Mody RR. Diffusion MR characteristics following concurrent radiochemotherapy predicts progression-free and overall survival in newly diagnosed glioblastoma. Tomography. 2015. 1: 37-43
6. Elson A, Paulson E, Bovi J, Siker M, Schultz C, Laviolette P. Evaluation of pre-radiotherapy apparent diffusion coefficient (ADC): Patterns of recurrence and survival outcomes analysis in patients treated for glioblastoma multiforme. J Neurooncol. 2015. 123: 179-88
7. Gupta A, Young RJ, Karimi S, Sood S, Zhang Z, Mo Q. Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. Am J Neuroradiol. 2011. 32: 1301-6
8. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005. 352: 997-1003
9. Ivanidze J, Lum M, Pisapia D, Magge R, Ramakrishna R, Kovanlikaya I. MRI features associated with TERT promoter mutation status in glioblastoma. J Neuroimaging. 2019. 29: 357-63
10. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987. 66: 865-74
11. Kikuchi Z, Shibahara I, Yamaki T, Yoshioka E, Shofuda T, Ohe R. TERT promoter mutation associated with multifocal phenotype and poor prognosis in patients with IDH wild-type glioblastoma. Neuro Oncol Adv. 2020. 2: vdaa114
12. Kinoshita M, Arita H, Goto T, Okita Y, Isohashi K, Watabe T. A novel PET index, 18F-FDG-11C-methionine uptake decoupling score, reflects glioma cell infiltration. J Nucl Med. 2012. 53: 1701-8
13. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
14. Li C, Wang S, Yan JL, Torheim T, Boonzaier NR, Sinha R. Characterizing tumor invasiveness of glioblastoma using multiparametric magnetic resonance imaging. J Neurosurg. 2019. 132: 1465-72
15. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection?. J Neurosurg. 2016. 124: 977-88
16. Liu J, Zhang X, Yan X, Sun M, Fan Y, Huang Y. Significance of TERT and ATRX mutations in glioma. Oncol Lett. 2019. 7: 95-102
17. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, FigarellaBranger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
18. Macerola E, Loggini B, Giannini R, Garavello G, Giordano M, Proietti A. Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 2015. 467: 177-84
19. Matsuda K, Kokubo Y, Kanemura Y, Kanoto M, Sonoda Y. Preoperative apparent diffusion coefficient of peritumoral lesion associate with recurrence in patients with glioblastoma. Neurol Med Chir (Tokyo). 2022. 62: 28-34
20. Nencha U, Rahimian A, Giry M, Sechi A, Mokhtari K, Polivka M. TERT promoter mutations and rs2853669 polymorphism: Prognostic impact and interactions with common alterations in glioblastomas. J Neurooncol. 2016. 126: 441-6
21. Nguyen HN, Lie A, Li T, Chowdhury R, Liu F, Ozer B. Human TERT promotor mutations enables survival advantage from MGMT promotor methylation in IDH1 wild-type primary glioblastoma treated by standard chemoradiotherapy. Nuero Oncol. 2017. 19: 34-404
22. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
23. Sasaki T, Fukai J, Kodama Y, Hirose T, Okita Y, Moriuchi S. Characteristics and outcomes of elderly patients with diffuse gliomas: A multi-institutional cohort study by Kansai molecular diagnosis network for CNS tumors. J Neurooncol. 2018. 140: 329-39
24. Shu C, Wang Q, Yan X, Wang J. The TERT promoter mutation status and MGMT promoter methylation status, combined with dichotomized MRI-derived and clinical features, predict adult primary glioblastoma survival. Cancer Med. 2018. 7: 3704-12
25. Simon M, Hosen I, Gousias K, Rachakonda S, Heidenreich B, Gessi M. TERT promotor mutations: A novel independent prognostic factor in primary glioblastomas. Neuro Oncol. 2015. 17: 45-52
26. Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001. 93: 1246-56
27. Spiegl-Kreinecker S, Lötsch D, Ghanim B, Pirker C, Mohr T, Laaber M. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro Oncol. 2015. 17: 1231-40
28. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
29. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Weller M, Fisher B. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009. 10: 459-66
30. Vivas-Buitrago T, Domingo RA, Tripathi S, De Biase G, Brown D, Akinduro OO. Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J Neurosurg. 2021. 136: 1-8
31. Wang K, Liu T, Ge N, Liu L, Yuan X, Liu J. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive cast PCR. Oncotarget. 2014. 5: 12428-39
32. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014. 32: 2718-26
33. Yamashita K, Hatae R, Hiwatashi A, Togao O, Kikuchi K, Momosaka D. Predicting TERT promoter mutation using MR imaged in patients with wild-type IDH1 glioblastoma. Diagn Interv Imaging. 2019. 100: 411-9
34. Yuan P, Cao JL, Abuduwufuer A, Wang LM, Yuan XS, Lv W. Clinical characteristics and prognostic significance of TERT promoter mutations in cancer: A cohort study and a meta-analysis. PLoS One. 2016. 11: e0146803