- Department of Neurosurgery, Dr. Moewardi General Hospital, Surakarta, Indonesia,
- Department of Neurosurgery, Fukuoka University Hospital, Fukuoka, Japan.
Correspondence Address:
Takashi Morishita, MD, PhD, Department of Neurosurgery, Fukuoka University Faculty of Medicine, Nanakuma 7-45-1, Jonan Ward, Fukuoka 814-0180, Japan.
DOI:10.25259/SNI_844_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Galih Indra Permana1, Takashi Morishita2, Hideaki Tanaka2, Ryuji Matsushita2, Hiromasa Kobayashi2, Hiroshi Abe2. Asymptomatic cable twisting in a patient with impending Twiddler syndrome detected during deep brain stimulation surgery for Parkinson’s disease: A case report. 15-Mar-2024;15:86
How to cite this URL: Galih Indra Permana1, Takashi Morishita2, Hideaki Tanaka2, Ryuji Matsushita2, Hiromasa Kobayashi2, Hiroshi Abe2. Asymptomatic cable twisting in a patient with impending Twiddler syndrome detected during deep brain stimulation surgery for Parkinson’s disease: A case report. 15-Mar-2024;15:86. Available from: https://surgicalneurologyint.com/surgicalint-articles/12802/
Abstract
Background: Deep brain stimulation (DBS) has consistently demonstrated high efficacy and safety in patients with Parkinson’s disease. Twiddler’s syndrome is a rare occurrence of hardware failure in patients undergoing neuromodulation. We report here a case of subclinical cable twisting jeopardizing Twiddler’s syndrome in a patient with Parkinson’s disease who underwent DBS surgery targeting the globus pallidus internus (GPI).
Case Description: A 70-year-old woman with a 7-year history of Parkinson’s disease refractory to medication was referred to our department for treatment of involuntary movements of the left hand and leg. She underwent right GPI DBS implantation. Left GPI DBS implantation was subsequently planned to manage resting tremors that developed in the right leg after the first surgery at around one year after the first surgery. During a routine check-up before the second surgery, we incidentally detected Twiddler’s syndrome. The patient showed no neurological deficits in the left extremities, the same as before right GPI DBS. We performed left GPI DBS concomitantly with the revision of the implantable pulse generator and extension wire.
Conclusion: Twiddler’s syndrome is a rare complication of DBS. Subclinical risk of cable twisting jeopardizing Twiddler’s syndrome is rarely detected without clinical indications of hardware failure. Neurosurgeons should be cognizant of and regularly monitor the implanted device in case serious complications occur.
Keywords: Deep brain stimulation, Parkinson’s disease, Twiddler’s syndrome
INTRODUCTION
Deep brain stimulation (DBS) is the treatment of choice for patients with movement disorders, including Parkinson’s disease, generalized dystonia, tremor, and Tourette syndrome. DBS has consistently demonstrated high efficacy and safety for movement disorders.[
Twiddler’s syndrome is a rare complication that occurs in patients undergoing neuromodulation with an implanted pacemaker or defibrillator[
CASE DESCRIPTION
A 70-year-old woman with a 7-year history of medication-refractory Parkinson’s disease was referred to our department for treatment of involuntary movements of the left hand and leg. She had no relevant psychiatric history, such as personal or family history of obsessive-compulsive disorder. She was independent in activities of daily living on medication; however, she was suffering from severe on/off motor fluctuations. Her preoperative neuropsychic evaluation was not significant for dementia or mental disorders. The Mini-Mental State Examination was 27/30, and the Montreal Cognitive Assessment was 27/30. Frontal Assessment Battery was 15/18. In addition, the Geriatric Depression Scale was 1/15, and the State and Trait Anxiety Inventory score was 31/80.
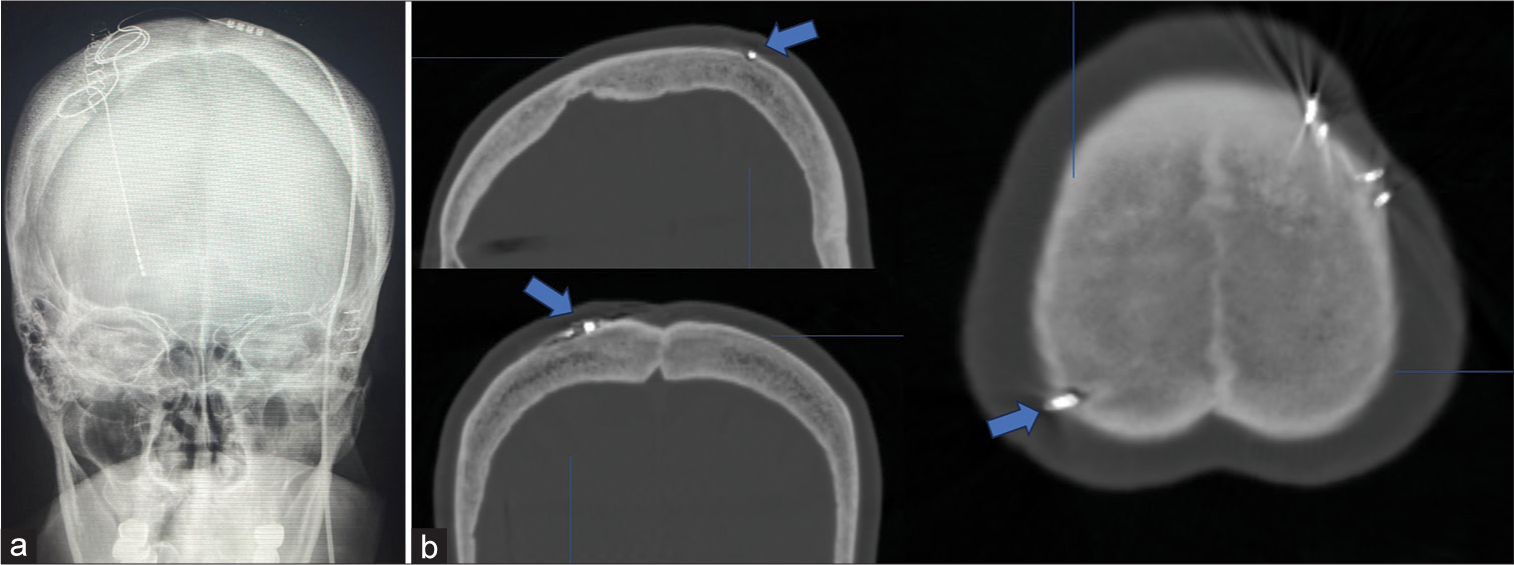
She underwent elective DBS targeting the right GPI. The DBS lead (model B33015, Medtronic, Minneapolis, MS) was implanted in the right GPI under local anesthesia. The implantable pulse generator (IPG; B35200, Medtronic) was implanted in the left subclavicular pouch without suture fixation under pectoral fascia on the same day under general anesthesia. Since the patient is right-handed, she requested to implant the IPG in the left subclavicular. A skull radiograph was performed immediately after surgery to confirm the location of the lead [
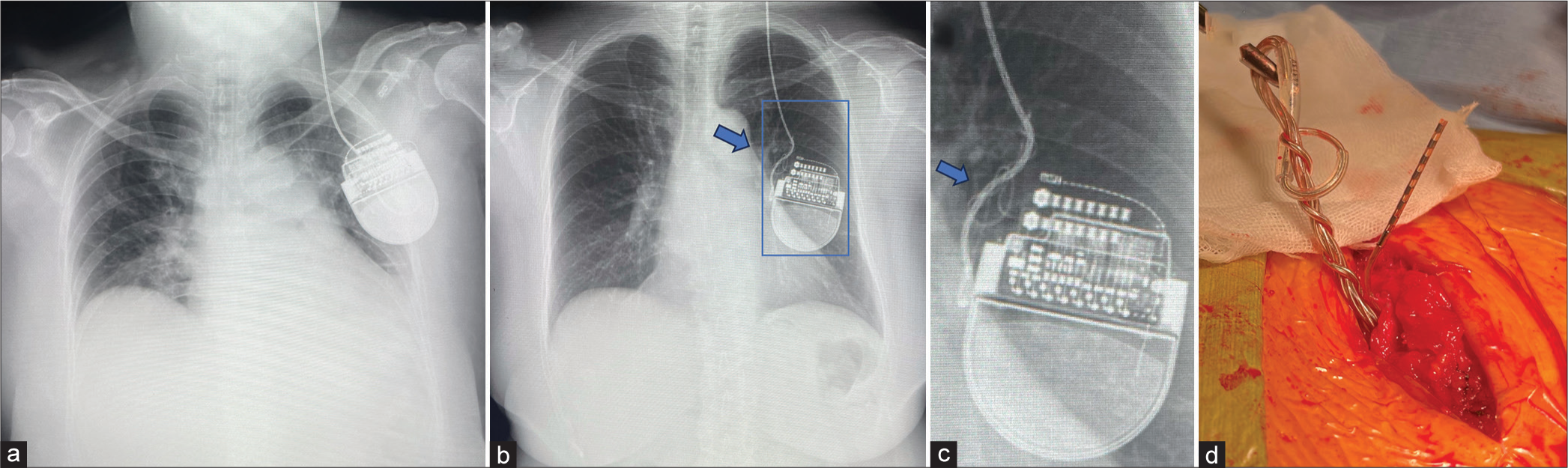
She had kept having benefits from the first DBS therapy and was then planned to undergo left GPI DBS for the right hemibody symptoms one year later. Around one year after DBS surgery, she developed a resting tremor in the right leg, and DBS implantation in the left GPI was planned. Before the surgery was performed. However, a chest X-ray during a routine check-up incidentally revealed that the extension wire was twisted in the chest cavity, and the IPG was flipped left to right [
Figure 2:
Images of the implantable pulse generator (IPG). (a) Chest radiographs showing the IPG in the left chest wall immediately postoperative and (arrows b and c) coiling of the extension wire around one year after deep brain stimulation surgery targeting the right globus pallidus internus. (c) A magnified image reveals the IPG has flipped left to right. (d) Intraoperative finding showing multiple coils in the extension wire near the IPG.
DISCUSSION
Although DBS of the GPI has been proven to be a safe and effective treatment for Parkinson’s disease, there is a risk of hardware, surgical, and/or stimulation complications.[
Our patient with Parkinson’s disease showed clinical improvement after right GPI DBS and did not recall having manipulated or moved the IPG intentionally. Identifying a tendency for the IPG to twist within the subcutaneous pocket is difficult, and it is unclear whether the movement disorder in Parkinson’s disease plays a contributing role. Neuropsychological and psychiatric profiles may not be able to detect any characteristics of Twiddler’s syndrome, which could include anxiety, dementia, depression, obsessive-compulsive behaviors, or paranoia. However, our patient had a risk factor of poorly fixed IPG in the loose subcutaneous space due to obesity.
The development of Twiddler’s syndrome is characterized by the recurrence of clinical symptoms and device failure resulting from manipulation or movement. Pain at the IPG site or along the extension wire path may accompany these clinical symptoms.[
Treatment of Twiddler’s syndrome usually involves surgical revision, fixation of the IPG, and the replacement of any damaged hardware. The IPG can be stabilized surgically to prevent it from twirling by fixing the IPG within a tight-fitting subcutaneous pocket using a nonabsorbable silk suture that is passed through the designated IPG hole and fastened to the muscle, fascia, or artificial pouch.[
CONCLUSION
Twiddler’s syndrome is a rare but serious complication of DBS resulting from IPG manipulations by the patient. Neurosurgeons should be mindful that the patient may subclinically manipulate IPG, and this may jeopardize DBS system dysfunction named Twiddler syndrome. Clinicians should carefully monitor the device in patients treated with DBS and be cognizant that it could be damaged during procedures or surgeries.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was supported in part by Grant-in-Aid for Scientific Research (C) (Grant numbers: 18K08956, 23K08555) from the Japan Society for the Promotion of Science, Takeda Science Foundation, and by Konishi Daiichi Hospital.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adams J, Shivkumar V. Twiddler’s Syndrome in deep brain stimulation. Mov Disord Clin Pract. 2020. 7: 859-60
2. Astradsson A, Schweder PM, Joint C, Green AL, Aziz TZ. Twiddler’s syndrome in a patient with a deep brain stimulation device for generalized dystonia. J Clin Neurosci. 2011. 18: 970-2
3. Bayliss CE, Beanlands DS, Baird RJ. The pacemaker-twiddler’s syndrome: A new complication of implantable transvenous pacemakers. Can Med Assoc J. 1968. 99: 371-3
4. Burdick AP, Okun MS, Haq IU, Ward HE, Bova F, Jacobson CE. Prevalence of Twiddler’s syndrome as a cause of deep brain stimulation hardware failure. Stereotact Funct Neurosurg. 2010. 88: 353-9
5. Geissinger G, Neal JH. Spontaneous Twiddler’s syndrome in a patient with a deep brain stimulator. Surg Neurol. 2007. 68: 454-6 discussion 456
6. Gelabert-Gonzalez M, Relova-Quinteiro JL, Castro-García A. “Twiddler syndrome” in two patients with deep brain stimulation. Acta Neurochir (Wien). 2010. 152: 489-91
7. Ghanchi H, Taka TM, Bernstein JE, Kashyap S, Ananda AK. The unsuccessful Twiddler: A case of Twiddler’s syndrome without deep brain stimulator lead breakage. Cureus. 2020. 12: e7786
8. Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: A review of the published literature. Stereotact Funct Neurosurg. 2006. 84: 248-51
9. Jackowiak E, Patil PG, Chou KL. The deep brain stimulation “twiddler syndrome”. JAMA Neurol. 2019. 76: 620
10. Liu X, Xu Y, Bergman H, Li S, Wang W. A systematic review of Twiddler’s syndrome: A hardware-related complication of deep brain stimulation. Neurosurg Rev. 2022. 45: 951-63
11. Morishita T, Hilliard JD, Okun MS, Neal D, Nestor KA, Peace D. Postoperative lead migration in deep brain stimulation surgery: Incidence, risk factors, and clinical impact. PLoS One. 2017. 12: e0183711
12. Paluzzi A, Belli A, Bain P, Liu X, Aziz TM. Operative and hardware complications of deep brain stimulation for movement disorders. Br J Neurosurg. 2006. 20: 290-5
13. Seijo FJ, Alvarez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson’s disease. Review of 272 procedures. Acta Neurochir (Wien). 2007. 149: 867-75 discussion 876
14. Silva PA, Chamadoira C, Costa H, Linhares P, Rosas MJ, Vaz R. Twiddler (or not) Syndrome: Questioning etiology for an uncommon form of hardware malfunction in deep brain stimulation. Surg Neurol Int. 2014. 5: S410-2
15. Tymchak Z, Vitali A. What’s the twist? Twiddler’s syndrome in deep brain stimulation. Can J Neurol Sci. 2017. 44: 726-7