- Unit of Neurosurgery, S. Elia Hospital, Caltanissetta, Italy
- Unit of Ophtalmology, S. Elia Hospital, Caltanissetta, Italy
- Neurosurgical Clinic, AOUP “Paolo Giaccone”, Post Graduate Residency Program in Neurologic Surgery, Department of Biomedicine Neurosciences and Advanced Diagnostics, School of Medicine, University of Palermo, Palermo, Italy
- Department of Neurosurgery, Highly Specialized Hospital of National Importance “Garibaldi”, Catania, Italy
Correspondence Address:
Salvatore Marrone, Unit of Neurosurgery, S. Elia Hospital, Caltanissetta, Italy.
DOI:10.25259/SNI_287_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Salvatore Marrone1, Corrado Pizzo2, Federica Paolini3, Evier Andrea Giovannini3, Antonio Crea4, Giovanni Cinquemani1, Rita Lipani1, Luca Ruggeri1, Jaime Mandelli1, Domenico Gerardo Iacopino3, Giuseppe Bona2, Luigi Basile1. Atypical Terson syndrome after subarachnoid hemorrhage from middle cerebral artery aneurysm rupture during coitus. 23-Aug-2024;15:291
How to cite this URL: Salvatore Marrone1, Corrado Pizzo2, Federica Paolini3, Evier Andrea Giovannini3, Antonio Crea4, Giovanni Cinquemani1, Rita Lipani1, Luca Ruggeri1, Jaime Mandelli1, Domenico Gerardo Iacopino3, Giuseppe Bona2, Luigi Basile1. Atypical Terson syndrome after subarachnoid hemorrhage from middle cerebral artery aneurysm rupture during coitus. 23-Aug-2024;15:291. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13064
Abstract
Background: Terson syndrome (TS) is a neuro-ophthalmologic disease arising due to subarachnoid hemorrhage (SAH), resulting in the formation of subhyaloid hemorrhagic spots. These spots can affect the ability to see due to the alteration of the optic cameras. Although it often affects both eyes, the symptoms and the eye involvement can be asymmetrical in rare cases.
Case Description: We described the case of a 52-year-old female patient who developed Terson disease following the rupture of a right middle cerebral artery aneurysm occurring during coitus with SAH (Fisher grade III). The aneurysm was treated by endovascular coiling. Interestingly, despite the major involvement of the right eye, the patient primarily manifested symptoms of visual changes in the left eye.
Conclusion: TS is a frequent ocular complication of SAH, with symptoms typically affecting both eyes. Characterized by hemorrhagic spots in both subhyaloid layers, the syndrome’s symptomatology is generally bilateral. However, in the case described, the manifestation is deemed atypical, primarily appearing contralateral to the hemisphere exhibiting a greater pattern of SAH.
Keywords: Terson syndrome, Subarachnoid hemorrhage, Middle cerebral artery aneurysms, Intracranial hypertension, Subhyaloid hemorrhage
INTRODUCTION
Terson syndrome (TS) is a disease of both ophthalmic and neurologic interest. It is characterized by intraocular hemorrhage (IOH), and it appears in 15–50% of patients with subarachnoid hemorrhage (SAH).[
Although it is most evident in aneurysmal SAHs, TS may also be an occasional finding in post-traumatic SAH, Moyamoya disease, or thrombosis of dural venous sinuses.[
CASE DESCRIPTION
A 52-year-old female patient presented with a prior history of autoimmune dermatitis and hypertension. During sex, she suffered a headache and blurred vision, followed by loss of consciousness. After regaining consciousness, she vomited and continued to complain about visual alterations with a stronger headache.
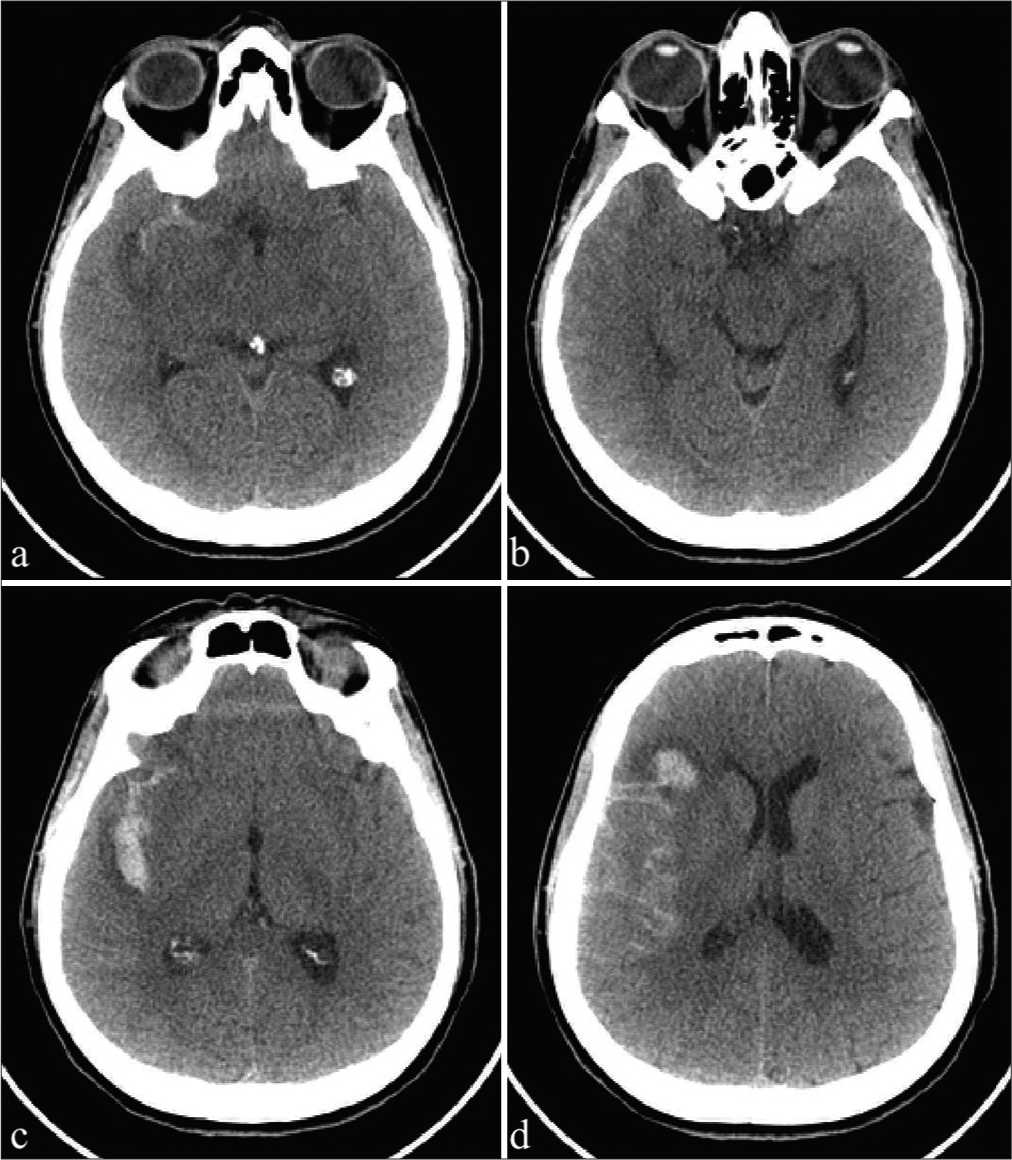
After her admission to our emergency room, she underwent a brain computed tomography scan, documenting diffuse Fisher type 3 SAH [
After the procedure, the patient was admitted to our hospital. Due to the persistence of visual alterations, the patient underwent a first ophthalmologic examination. Tropicamide 1% was instilled, and through binocular indirect ophthalmoscopy, subhyaloid hemorrhage was detected in both the eyes in the macular region (bigger in the right eye than the left one) and vitreous hemorrhage in the left eye [
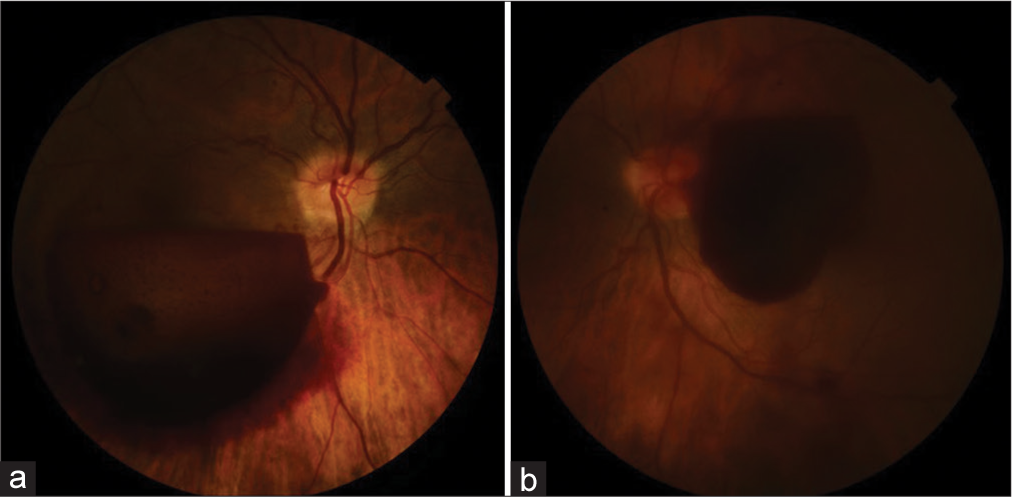
Figure 3:
(a) Right eye: Retrohyaloid hemorrhage covering part of the macula and mild papilledema (optic disk swelling). The patient complained of decreased vision. (b) Left eye: Retrohyaloid hemorrhage covering the fovea and vitreous hemorrhage. The patient complained of blurred and decreased vision.
Medical therapy with tranexamic acid was prescribed. 1 week later, the patient referred an improved of her vision, that was less blurred. A second examination was performed. Her best-corrected visual acuity was 2/10 on the right eye and 10/10 on the left one. The last ocular examination showed the presence of hyper-refractive material beneath the hyaloid, which masked part of the posterior pole in the right eye and just a little part of the macula on the left one. Besides, on the right eye, ocular tests detected the presence of hyper-refractive fog over the fovea. This could explain the reason why the patient complained about right blurred vision, even if TS involved both eyes and vitreous hemorrhage was found only in the left eye.
DISCUSSION
TS is a rare ophthalmologic disorder characterized by vitreoretinal hemorrhage secondary to blood expansion into the subarachnoid space and rapid intracranial pressure (ICP).[
During intercourse, physiological phenomena are known to result in a transient change of balance between sympathetic and parasympathetic systems. It leads to increased blood pressure that is typically transient in healthy subjects, but it can develop persistently in patients with long-lasting history of hypertension.[
Although in literature there is no strict relation between the SAH side and the eye affected by TS, it seems to occur bilaterally or unilaterally on the same aneurysm side.[
A possible explanation of our case is based on the new venous hypertension theory.[
CONCLUSION
TS is a rare condition characterized by hemovitreous extending even into the peri-retinal region and is associated with neurosurgical hemorrhagic disorders such as SAH from ruptured cerebral aneurysm. The exact etiopathogenesis is doubtful, but processes related to IH affecting venous and glymphatic drainage would be the main reasons. Unlike the typical forms, atypical forms may not be preceded by clinical and radiological signs of IH. We described an unusual case of fundoscopically evident bilateral TS in a patient with intercourse rupture of the right MCA aneurysm, but symptomatic only in the contralateral eye despite the major pattern of blood spillage was present in the right subarachnoid space and although angiographic vasospasm signs were evident in the M1 segment and the anterior circulation of the right hemisphere.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aboulhosn R, Raju B, Jumah F, Majmundar N, Prenner J, Matin T. Terson’s syndrome, the current concepts and management strategies: A review of literature. Clin Neurol Neurosurg. 2021. 210: 107008
2. Axier A, Rexiati N, Wang Z, Cheng X, Su R, Aikeremu R. Effect of hemodynamic changes on the risk of intracranial aneurysm rupture: A systematic review and meta-analysis. Am J Transl Res. 2022. 14: 4638-47
3. Chai CZ, Ho UC, Kuo LT. Systemic Inflammation after aneurysmal subarachnoid hemorrhage. Int J Mol Sci. 2023. 24: 10943
4. Citirik M, Tekin K, Teke MY. Terson syndrome with persistent vitreous hemorrhage following traumatic brain injury. Saudi J Ophthalmol. 2019. 33: 392-7
5. Doshi R, Neil-Dwyer G. A clinicopathological study of patients following a subarachnoid hemorrhage. J Neurosurg. 1980. 52: 295-301
6. Hayreh SS. Pathogenesis of Terson syndrome. Indian J Ophthalmol. 2022. 70: 4130-7
7. Inoue T, Tsutsumi K, Shigeeda T. Terson’s syndrome as the initial symptom of subarachnoid hemorrhage caused by ruptured vertebral artery aneurysm-case report. Neurol Med Chir (Tokyo). 2006. 46: 344-7
8. Juvela S. Growth and rupture of unruptured intracranial aneurysms. J Neurosurg. 2019. 131: 843-51
9. Kassubek R, Weinstock D, Behler A, Müller HP, Dupuis L, Kassubek J. Morphological alterations of the hypothalamus in idiopathic intracranial hypertension. Ther Adv Chronic Dis. 2022. 13: 204062232211413
10. Kuhn F, Morris R, Witherspoon CD, Mester V. Terson syndrome. Ophthalmology. 1998. 105: 472-7
11. Lee JY, Kim DY, Lee HJ, Jeong JH, Park SP, Kim JY. Atypical acute retinal necrosis accompanied by Terson’s syndrome: A case report. BMC Ophthalmol. 2017. 17: 255
12. Ma J, Zheng Y, Li P, Zhou T, Sun Z, Ju T. Risk factors for the rupture of intracranial aneurysms: A systematic review and meta-analysis. Front Neurol. 2023. 14: 1268438
13. Medele RJ, Stummer W, Mueller AJ, Steiger HJ, Reulen HJ. Terson’s syndrome in subarachnoid hemorrhage and severe brain injury accompanied by acutely raised intracranial pressure. J Neurosurg. 1998. 88: 851-4
14. Møllgård K, Beinlich FR, Kusk P, Miyakoshi LM, Delle C, Plá V. A mesothelium divides the subarachnoid space into functional compartments. Science (1979). 2023. 379: 84-8
15. Moss HE. Retinal vein changes as a biomarker to guide diagnosis and management of elevated intracranial pressure. Front Neurol. 2021. 12: 751370
16. Mulholland DA, Page B. A cautionary tale: Terson’s syndrome presenting to eye casualty. Eye. 1997. 11: 121-2
17. Murthy S, Salas D, Hirekataur S, Ram R. Terson’s syndrome presenting as an ophthalmic emergency. Acta Ophthalmol Scand. 2002. 80: 665-6
18. Ogawa T, Kitaoka T, Dake Y, Amemiya T. Terson syndrome. Ophthalmology. 2001. 108: 1654-6
19. Pfausler B, Belcl R, Metzler R, Mohsenipour I, Schmutzhard E. Terson’s syndrome in spontaneous subarachnoid hemorrhage: A prospective study in 60 consecutive patients. J Neurosurg. 1996. 85: 392-4
20. Pluta RM, Butman JA, Schatlo B, Johnson DL, Oldfield EH. Subarachnoid hemorrhage and the distribution of drugs delivered into the cerebrospinal fluid. J Neurosurg. 2009. 111: 1001-7
21. Raevis J, Elmalem VI. Pseudotumor cerebri syndrome causing a terson like syndrome. Am J Ophthalmol Case Rep. 2020. 20: 100993
22. Reynolds MR, Willie JT, Zipfel GJ, Dacey RG. Sexual intercourse and cerebral aneurysmal rupture: Potential mechanisms and precipitants. J Neurosurg. 2011. 114: 969-77
23. Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: A systematic review. Neurocrit Care. 2005. 2: 110-8
24. Saboori P, Sadegh A. Histology and morphology of the brain subarachnoid trabeculae. Anat Res Int. 2015. 2015: 279814
25. Sánchez Ferreiro AV, Muñoz Bellido L. Atypical presentation of Terson syndrome: Presentation of a case. Neurología. 2012. 27: 380-1
26. Sánchez-Vicente JL, Frau-Aguilera L, Sánchez-Vicente P, Herrador-Montiel A, Rueda-Rueda T, Castilla-Lázpita A. Atrofia macular en el síndrome de Terson. Arch Soc Esp Oftalmol. 2015. 90: 26-9
27. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage. JAMA. 2007. 298: 1429
28. Subbiah S, Wilson S, Best R. An unusual presentation of Terson’s syndrome. Eye. 2007. 21: 855-6
29. Szeligowski T, Fu DJ, Fernandez-Ledo N, Birtel J, Aslam SA, Patel CK. Photoreceptor damage in Terson Syndrome. Retina. 2023. 43: 1557-62
30. Takeuchi T, Kasahara TE, Iwasaki M, Iwasaki T. Terson’s syndrome which arose from ruptured right superior cerebellar artery aneurysm: A case report and 32 series investigations. No Shinkei Geka. 1997. 25: 259-64
31. Tan H, Yang W, Wu C, Liu B, Lu H, Wang H. Assessment of the role of intracranial hypertension and stress on hippocampal cell apoptosis and hypothalamic-pituitary dysfunction after TBI. Sci Rep. 2017. 7: 3805
32. Toro MD, Castellino N, Russo A, Scollo D, Avitabile T, Rejdak R. Optic nerve head and retinal changes in idiopathic intracranial hypertension: Correlation with short-term cerebrospinal fluid pressure monitoring. J Clin Med. 2024. 13: 562
33. Vosoughi AR, Micieli JA. Vitreous hemorrhage as the presenting sign of idiopathic intracranial hypertension. Case Rep Ophthalmol. 2022. 13: 896-900
34. Wu F, Liu Z, Li G, Zhou L, Huang K, Wu Z. Inflammation and oxidative stress: Potential targets for improving prognosis after subarachnoid hemorrhage. Front Cell Neurosci. 2021. 15: 739506
35. Xue-Rui T, Ying L, Da-Zhong Y, Xiao-Jun C. Changes of blood pressure and heart rate during sexual activity in healthy adults. Blood Press Monit. 2008. 13: 211-7