- Department of Neurosurgery, Manchester Center for Clinical Neurosciences, Salford, United Kingdom.
- Department of Neuropsyhology, Manchester Center for Clinical Neurosciences, Salford, United Kingdom.
- Department of Anesthesiology, Manchester Center for Clinical Neurosciences, Salford, United Kingdom.
Correspondence Address:
Evangelos Drosos, Department of Neurosurgery, Manchester Center for Clinical Neurosciences, Salford, United Kingdom.
DOI:10.25259/SNI_719_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Evangelos Drosos1, Helen Maye1, Amir Saam Youshani1, Sheeba Ehsan2, Cally Burnand3, Pietro Ivo D’Urso1. Awake brain surgery for autistic patients: Is it possible?. 18-Nov-2022;13:543
How to cite this URL: Evangelos Drosos1, Helen Maye1, Amir Saam Youshani1, Sheeba Ehsan2, Cally Burnand3, Pietro Ivo D’Urso1. Awake brain surgery for autistic patients: Is it possible?. 18-Nov-2022;13:543. Available from: https://surgicalneurologyint.com/surgicalint-articles/12017/
Abstract
Background: Awake neurosurgery is currently the mainstay for eloquent brain lesions. Opting for an awake operation is affected by a number of patient-related factors. We present a case of a patient with autistic spectrum disorder (ASD) that was successfully operated for a brain tumor through awake craniotomy. To the best of our knowledge, this is the first reported case in the literature.
Case Description: A 42-year-old patient, with known ASD since his childhood, underwent awake craniotomy for a left supplementary motor area tumor. Detailed preoperative preparation of the patient was done to identify special requirements and establish a good patient-team relationship. Intraoperatively, continuous language and motor testing were performed. Conversation and music were the main distractors used. Throughout the operation, the patient remained calm and cooperative, even during a focal seizure. Mapping allowed for >80% resection of the tumor. Postoperatively, the patient recovered without any deficits.
Conclusion: This case shows that with growing experience and meticulous preparation, the limits of awake craniotomy can be expanded to include more patients that were previously considered unfit.
Keywords: Autism, Autistic spectrum disorder, Awake craniotomy, Brain mapping, Glioma, Neuro-oncology
INTRODUCTION
Awake neurosurgery for eloquent brain areas was first introduced by Penfield. During the 1980s, it was popularized by Ojeman and is now the gold-standard treatment for lesions within, or near suspected eloquent areas.[
A fundamental principle for a good functional outcome is careful patient selection. A number of patient-related factors have been considered relative or absolute contraindications. Significant neurological deficit, such as moderate to severe speech disturbances, profound motor weakness, emotional or psychiatric disorders may undermine the patient’s intraoperative cooperation. Other factors such as learning difficulties, seizures, obesity (causing airway restrictions), or young age have also been raised as relative contraindications.[
Autistic spectrum disorder (ASD) involves a multitude of symptoms and varies in severity. The classic triad includes impairment of social communication, social skills, and behavioral flexibility.[
We present the case of a patient with ASD who underwent resection of a left frontal glioma through awake craniotomy. To the best of our knowledge, this is the first report in the literature.
CASE DESCRIPTION
Patient information
A 42-year-old right-handed patient presented with seizures, manifesting as vacant episodes with postictal headache, dizziness, and vomiting. From his medical history, a seizure was reported at the age of 7. Developmentally, he first walked at 16 months old and spoke later than that. Diagnosis of ASD was set after primary school. Main initial components were learning difficulties and a mild struggle socializing with children. Psychology assessment at that age diagnosed all three main aspects of ASD. Mainstream school schedule was considered not suitable based on his performance, leading him to attend a special secondary school, and, subsequently, adjusted college admission examinations. During his adult life, he lived with his mother, his primary carer, on whom he was highly dependent and worked at a voluntary placement.
Clinical presentation
On presentation, his speech was mildly hesitant, with signs of anxiety.
Diagnostic assessment
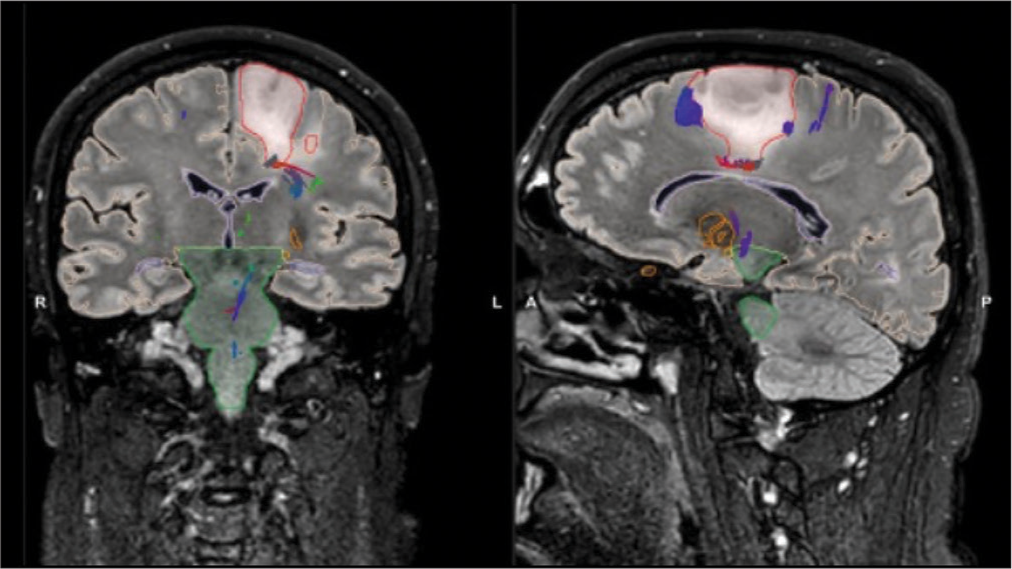
Magnetic resonance imaging (MRI) showed a 3cm, noncontrast enhancing tumor of the mid-posterior part of the left superior frontal gyrus. The tumor mainly invaded the anatomical region of the supplementary motor area (SMA), extending inferiorly to the cingulate gyrus [
Figure 1:
Preoperative T2 FLAIR magnetic resonance imaging (MRI) scan in coronal (left) and sagittal (right) cuts. Tumor margins illustrated during preoperative planning in red. The tumor (red) is located in the supplementary motor area, with signs of extension to the cingulate gyrus. At the posterolateral border, tumor tractography shows corticospinal tract fibers (purple). (green: Brainstem, orange: Anterior commisure, yellow: Optic radiation).
Initial neuropsychology assessment focused on the patient’s communication and understanding of the process, for which he demonstrated high level of insight. His anxiety was noted to respond well to distraction and instruction. His comprehension, counting, and ability to follow instructions were normal. His mood was stable, with no variations during the day.
After discussion at the multidisciplinary team meeting, surgical resection through awake craniotomy was offered.
Preoperative anesthetic assessment did not raise any major concerns. The positioning and various steps of the surgery were explained in detail to him and his mother. The patient seemed to fully understand and was confident that he would be able to cope, mentioning he would use music to calm him down, as well as having people talking to him.
Aiming to maintain his baseline functional status, it was deemed necessary to avoid damaging SMA functional cortex, while ensuring safe maximal resection. Therefore, an awake craniotomy was planned with adjuncts: 5-Aminolevulinic acid (5-ALA), intraoperative monitoring (IOM), and intraoperative ultrasound (iUS).
Surgery
The patient was positioned supine with the head rotated 30 degrees to the right, resting on a horseshoe to avoid discomfort from the pin-fixation. After positioning, light sedation with dexmedetomidine was introduced and electrodes for intraoperative electroencephalography and motor-evoked potentials were inserted. Scalp blocks were performed and additional bupivacaine/lidocaine/ adrenaline infiltration of the wound was performed after prepping and draping. Frameless magnetic image guidance system (STEALTH-AXIEM MRI registration, ©Medtronic) was used.
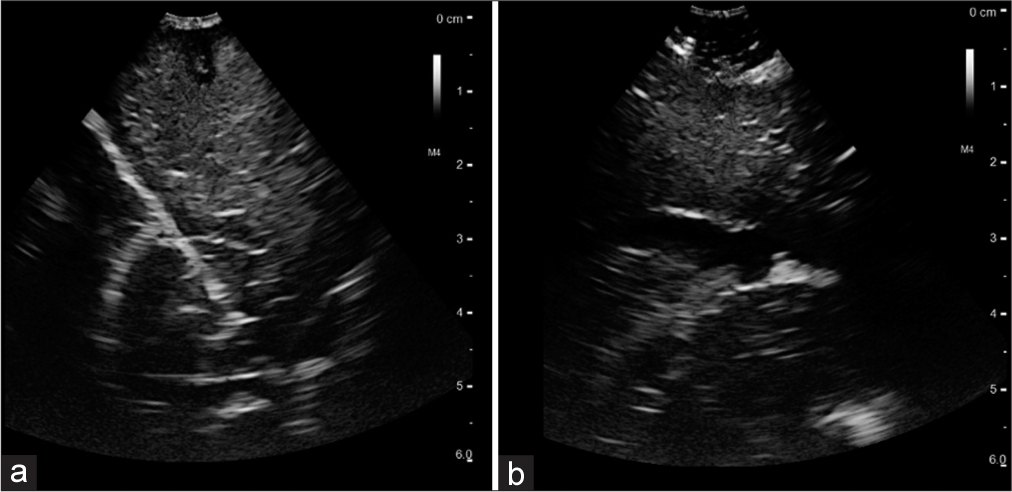
A 2 × 2.5cm left frontal parasagittal mini-craniotomy was performed, through a 4 cm left coronal incision. On dura opening, tumor borders were identified with iUS [
An area of cortical abnormality was identified and utilized as entry point for internal debulking of the tumor. The tumor was soft and easily resected by ultrasonic aspirator and suction.
Figure 2:
(a) Intraoperative ultrasound after dural opening. Hyperechoic lesion measuring 3 cm in depth. On the left, the falx is recognized by its bright white hyperechoic signal and its shape. (b) View of the posterolateral part of the resection cavity at the end of the operation. Hyperechoic residual tumor at 2 cm depth can be identified. At this area, subcortical stimulation identified the corticospinal tract.
Resection was guided by subcortical bipolar stimulation. Posterolaterally, the internal capsule fibers were identified by IOM at 10 mAmp and resection was stopped at 2 mAmp stimulation. Anteriorly, resection was discontinued when stimulation resulted in speech errors. Medially and inferiorly subpial dissection was followed to the falx and the cingulate gyrus. A single focal seizure was experienced by the patient, causing hand jerking with spikes on ECoG and was easily controlled with ice-cold saline irrigation.
Intraoperative neuropsychological testing included motor function and multitasking, with fine movement and 9-peg hole test, as well as hand-arm-leg movement. Speech was tested utilizing counting, free speech, and the Stroop test. No specific adaptations were required to the intraoperative tests.
At the end, blue light was utilized to identify 5-ALA enhancing tumor in the noneloquent parts of the resection cavity. Final iUS, highlighted margins of resection, confirmed with image guidance [
Throughout the operation, the patient was calm and co-operative. Free speech was tested periodically by the neuropsychologist and anesthetist by a continuous conversation with him about his life and habits. A playlist with his favorite music had been prepared and played at low intensity in the background perioperatively, to keep him distracted and calm. No further adaptation to the protocol was required.
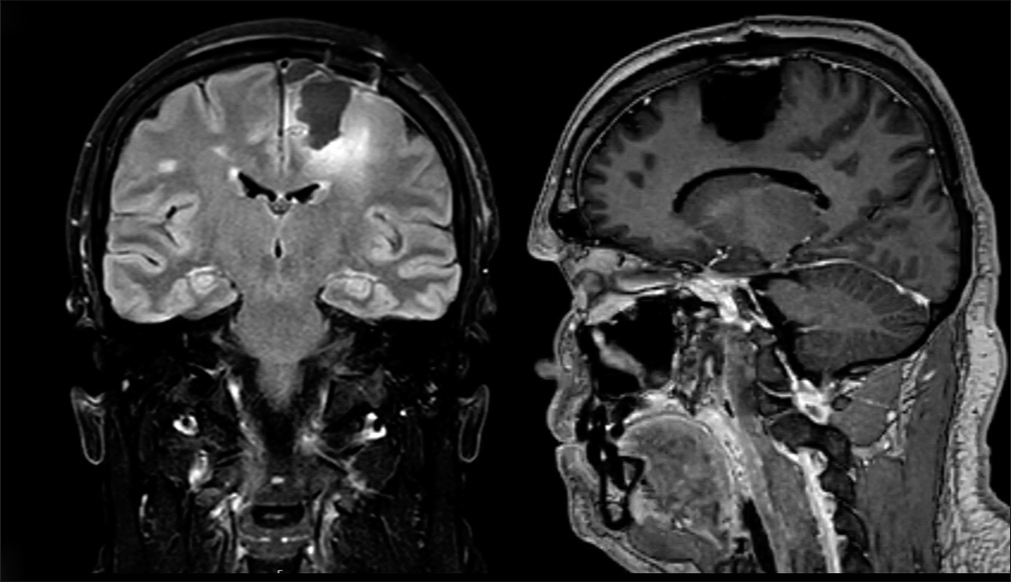
Recovery was uneventful, without further seizures. His mother stayed with him overnight to assist him with the hospital environment and was discharged on the 3rd postoperative day without new neurological symptoms or deficits. Postoperative MRI confirmed 81% resection of the tumor (26.8 cm3 vs. 5.2 cm3) [
Follow-up and outcomes
Histological diagnosis was consistent with diffuse astrocytoma, IDH mutant, and the WHO grade III. The patient is currently undergoing chemoradiotherapy. No new neurological or neuropsychology symptoms have been identified on subsequent follow-up.
DISCUSSION
Awake cranial surgery has been established as the mainstay for lesions involving or in close proximity to eloquent areas of the brain.[
The commonest protocols are the “awake throughout” and the “asleep-awake-asleep.” As the name implies, the patient can either be awake or receive mild sedation for the first part of the operation; or they can be fully sedated and intubated for the first and in some cases for the last part of the operation, respectively. In both protocols, the goal is to maintain the patient fully awake, calm, pain-free, and able to co-operate during the mapping and resection of the lesion.[
One of the pillars for successful intraoperative mapping is the co-operation between the patient and the team. Patients, very commonly, report anxiety before an awake craniotomy. In our case, the first step was to minimize this thorough preoperative discussion about the operation and the patient’s role, as well as adequate preparation for the intraoperative tests.[
Typical characteristics of ASD are compulsive behaviors, difficulty in concentration, irritability, and, sometimes, aggression.[
In our case, the patient had a relatively good functional status, requiring an in-house carer (in this case his mother) assisting with daily needs. He was thoroughly informed about what would happen on the day of surgery on multiple occasions preoperatively and had a detailed discussion with all members of the team. This created continuity and clear anticipation of the stages, reducing his anxiety. Furthermore, he was able to express his specific requirements; based on the coping mechanisms, he had developed throughout his life. Having the anesthetist and neuropsychologist engaging with him in casual conversation and listening to his favorite music were special requests that he had made during the preoperative preparation. All this led to effective co-operation even during the event of the epileptic seizure, allowing maximal tumor excision within functional limits.
CONCLUSION
Continual growing experience, established protocols and ongoing understanding of brain functions, encourages our ability to push the boundaries of awake brain surgery. This case shows that patients with mild intellectual or emotional issues, who would be considered unsuitable candidates in the past, can be safely operated awake, as long as the basic principles are followed, thorough preoperative preparation and a good patient-team relationship are established.
Ethics
The patient has signed the generic consent form, according to the Trust’s policy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
All authors contributed to the conceptualization, data collection, drafting, and confirmation of the final version of the manuscript.
References
1. Burnand C, Sebastian J. Anaesthesia for awake craniotomy. Continuing Educ Anaesth Crit Care Pain. 2013. 14: 6-11
2. Burnand C, Sebastian J. Survey of anaesthesia for awake craniotomy. J Neurosurg Anesthesiol. 2012. 24: 249
3. Cashin A, Sci DA, Barker P. The triad of impairment in autism revisited. J Child Adolesc Psychiatr Nurs. 2009. 22: 189-93
4. Geretsegger M, Elefant C, Mössler KA, Gold C. Music therapy for people with autism spectrum disorder. Cochrane Database Syst Rev. 2014. 2014: CD004381
5. Hejrati N, Spieler D, Samuel R, Regli L, Weyerbrock A, Surbeck W. Conscious experience and psychological consequences of awake craniotomy. World Neurosurg. 2019. 129: e381-6
6. Kim SH, Choi SH. Anesthetic considerations for awake craniotomy. Anesth Pain Med (Seoul). 2020. 15: 269-74
7. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018. 392: 508-20
8. Morshed RA, Young JS, Lee AT, Berger MS, Hervey-Jumper SL. Clinical pearls and methods for intraoperative awake language mapping. Neurosurgery. 2021. 89: 143-53
9. Nabavi A, Goebel S, Doerner L, Warneke N, Ulmer S, Mehdorn M. Awake craniotomy and intraoperative magnetic resonance imaging: Patient selection, preparation, and technique. Top Magn Reson Imaging. 2009. 19: 191-6
10. Riquin E, Dinomais M, Malka J, Lehousse T, Duverger P, Menei P. Psychiatric and psychologic impact of surgery while awake in children for resection of brain tumors. World Neurosurg. 2017. 102: 400-5
11. Sewell D, Smith M. Awake craniotomy: Anesthetic considerations based on outcome evidence. Curr Opin Anaesthesiol. 2019. 32: 546-52
12. Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta-analysis. J Autism Dev Disord. 2014. 44: 627-35
13. Venkatraghavan L, Bharadwaj S, Au K, Bernstein M, Manninen P. Same-day discharge after craniotomy for supratentorial tumour surgery: A retrospective observational single-centre study. Can J Anaesth. 2016. 63: 1245-57