- Department of Neurosurgery, Osaka University, Suita, Japan.
- Department of Neurosurgery, Osaka University Graduate School of Medicine, Suita, Japan.
Correspondence Address:
Noriyuki Kijima, Department of Neurosurgery, Osaka University, Suita,
DOI:10.25259/SNI_52_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Akihiro Yamamoto1, Noriyuki Kijima1, Reina Utsugi1, Koki Mrakami1, Hideki Kuroda1, Tetsuro Tachi1, Ryuichi Hirayama1, Yoshiko Okita1, Naoki Kagawa2, Haruhiko Kishima1. Awake surgery for a deaf patient using sign language: A case report. 24-May-2024;15:167
How to cite this URL: Akihiro Yamamoto1, Noriyuki Kijima1, Reina Utsugi1, Koki Mrakami1, Hideki Kuroda1, Tetsuro Tachi1, Ryuichi Hirayama1, Yoshiko Okita1, Naoki Kagawa2, Haruhiko Kishima1. Awake surgery for a deaf patient using sign language: A case report. 24-May-2024;15:167. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12912
Abstract
Background: Although awake surgery is the gold standard for resecting brain tumors in eloquent regions, patients with hearing impairment require special consideration during intraoperative tasks.
Case Description: We present a case of awake surgery using sign language in a 45-year-old right-handed native male patient with hearing impairment and a neoplastic lesion in the left frontal lobe, pars triangularis (suspected to be a low-grade glioma). The patient primarily communicated through sign language and writing but was able to speak at a sufficiently audible level through childhood training. Although the patient remained asymptomatic, the tumors gradually grew in size. Awake surgery was performed for tumors resection. After the craniotomy, the patient was awake, and brain function mapping was performed using tasks such as counting, picture naming, and reading. A sign language-proficient nurse facilitated communication using sign language and the patient vocally responded. Intraoperative tasks proceeded smoothly without speech arrest or verbal comprehension difficulties during electrical stimulation of the tumor-adjacent areas. Gross total tumor resection was achieved, and the patient exhibited no apparent complications. Pathological examination revealed a World Health Organization grade II oligodendroglioma with an isocitrate dehydrogenase one mutant and 1p 19q codeletion.
Conclusion: Since the patient in this case had no dysphonia due to training from childhood, the task was presented in sign language, and the patient responded vocally, which enabled a safe operation. Regarding awake surgery in patients with hearing impairment, safe tumor resection can be achieved by performing intraoperative tasks depending on the degree of hearing impairment and dysphonia.
Keywords: Awake surgery, Deaf patient, Low-grade glioma
INTRODUCTION
Awake craniotomy for brain tumor resection is the gold standard for resecting low-grade gliomas located in eloquent areas.[
Hearing impairment is divided into two categories: pre- and postlingual. Prelingual hearing impairment is either present at birth or begins before language is acquired, usually around five years of age. Postlingual hearing impairment occurs after speech development. Hereditary and environmental factors cause hearing impairment.[
CASE DESCRIPTION
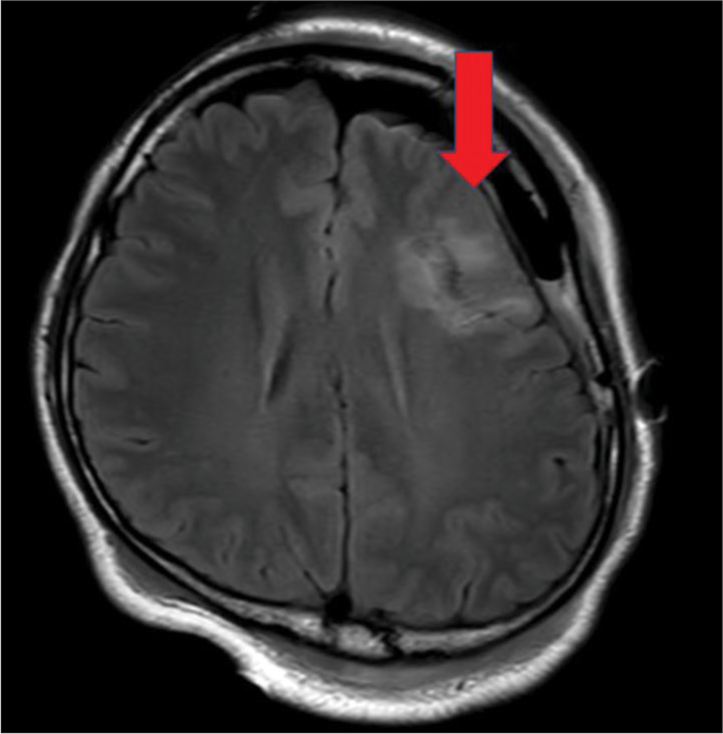
The patient was a 45-year-old right-handed native man with deafness. The patient presented with a mild headache seven years before being referred to our institution. Magnetic resonance imaging (MRI) revealed a non-enhanced, homogeneous lesion with a 25 mm diameter in the left frontal lobe (pars triangularis) [
The tumor was located in the left frontal lobe; we decided to perform an awake craniotomy to preserve verbal function. A few days before the awake craniotomy, we presented the patient with an intraoperative task, including number counting, picture naming, reading Japanese characters, bending, and stretching the right elbow. A nurse with sign language skills confirmed that the patient could vocally complete these tasks. On the day of the awake craniotomy, the patient was placed in the right lateral position with his head fixed with a Mayfield pin holder after induction of general anesthesia. We performed a left frontotemporal craniotomy under general anesthesia. The patient was awakened after the brain surface was exposed, wherein bipolar stimulation (NIM Eclipse, Medtronic, Dublin, Ireland) was performed for cortical mapping. Brain function mapping was performed using tasks such as number counting, picture naming, and reading Japanese characters. The nurse with sign language skills presented tasks and asked questions in sign language that the patient vocally answered. We ensured that the patient’s comfort and pain were adequately controlled by frequent communication using sign language during intraoperative mapping and tumor removal because the patient had difficulty expressing his discomfort; the patient’s face was hidden behind the drapes.
Figure 4:
Intraoperative pictures (a) before and (b) after tumor resection. The brain surface directly above the tumor, surrounded by “A,” “B,” “C,” and “D” markers, was edematous and had a color different from that of the surrounding area. No speech arrest or motor deficit was observed with electrical stimulation to the tumor surface and premotor area (“1” marker).
After tumor removal, the patient we re-intubated the patient during dural closure, bone flap fixation, and skin suturing. Postoperative MRI revealed no apparent residual tumors [
DISCUSSION
Here, we describe a case of awake craniotomy using sign language in a patient with hearing impairment with oligodendroglioma. Four previous studies have reported cases of awake craniotomy using sign language in patients with hearing impairment for glioma resection.[
Our case is different from these previous reports in that the patient learned to speak from childhood, whereas the case reported by Lau et al. is similar to our case in that the patient learned to speak vocally.[
Our patient did not exhibit any neurological signs on electrical stimulation during awake craniotomy, whether this was because the tumor had already replaced language function or if the patient had a different language pathway from their childhood, which is unclear.
The previous studies on deaf signers reported that language organization was similar to that in patients without deafness [
CONCLUSION
Here, we report a case of awake craniotomy using sign language in a patient with hearing impairment. Awake craniotomy in patients with hearing impairment can be successfully performed through intraoperative tasks. However, each individual has a different degree of hearing impairment and dysphonia. In addition, whether patients with hearing impairment could have similar language processing pathways compared with those without deafness is unclear. Therefore, adjusting the most appropriate intraoperative tasks depending on each individual is crucial.
Ethical approval
The research/study approved by the Institutional Review Board at Osaka University, number 22302, dated October 18, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chen PA, Chen YC, Wei KC, Chen KT. Awake craniotomy for a left pan-hippocampal diffuse low-grade glioma in a deaf and mute patient using sign language. World Neurosurg. 2020. 134: 629-34
2. Corina DP, McBurney SL, Dodrill C, Hinshaw K, Brinkley J, Ojemann G. Functional roles of Broca’s area and SMG: Evidence from cortical stimulation mapping in a deaf signer. Neuroimage. 1999. 10: 570-81
3. Duffau H. Is non-awake surgery for supratentorial adult low-grade glioma treatment still feasible?. Neurosurg Rev. 2018. 41: 133-9
4. Emmorey K. Sign language: How the brain represents phonology without sound. Curr Biol. 2020. 30: R1361-3
5. Gogos AJ, Young JS, Morshed RA, Hervey-Jumper SL, Berger MS. Awake glioma surgery: Technical evolution and nuances. J Neurooncol. 2020. 147: 515-24
6. Ito T, Noguchi Y, Yashima T, Ohno K, Kitamura K. Hereditary hearing loss and deafness genes in Japan. J Med Dent Sci. 2010. 57: 1-10
7. Kassubek J, Hickok G, Erhard P. Involvement of classical anterior and posterior language areas in sign language production, as investigated by 4 T functional magnetic resonance imaging. Neurosci Lett. 2004. 364: 168-72
8. Lau R, Malhotra AK, McAndrews MP, Kongkham P. Subcortical language localization using sign language and awake craniotomy for dominant posterior temporal glioma resection in a hearing-impaired patient. Acta Neurochir (Wien). 2023. 165: 1665-9
9. Leonard MK, Lucas B, Blau S, Corina DP, Chang EF. Cortical encoding of manual articulatory and linguistic features in American Sign Language. Curr Biol. 2020. 30: 4342-51
10. MacSweeney M, Capek CM, Campbell R, Woll B. The signing brain: The neurobiology of sign language. Trends Cogn Sci. 2008. 12: 432-40
11. Martino J, Velasquez C, Vázquez-Bourgon J, de Lucas EM, Gomez E. Cross-modal recruitment of auditory and orofacial areas during sign language in a deaf subject. World Neurosurg. 2017. 105: 1033.e1-5
12. Metellus P, Boussen S, Guye M, Trebuchon A. Successful insular glioma removal in a deaf signer patient during an awake craniotomy procedure. World Neurosurg. 2017. 98: 883.e1-5
13. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008. 358: 18-27
14. Wiggin M, Sedey AL, Yoshinaga-Itano C, Mason CA, Gaffney M, Chung W. Frequency of early intervention sessions and vocabulary skills in children with hearing loss. J Clin Med. 2021. 10: 5025
15. Wrightson AS. Universal newborn hearing screening. Am Fam Physician. 2007. 75: 1349-52
16. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early-and later-identified children with hearing loss. Pediatrics. 1998. 102: 1161-71
17. Zhang JJ, Lee KS, Voisin MR, Hervey-Jumper SL, Berger MS, Zadeh G. Awake craniotomy for resection of supratentorial glioblastoma: A systematic review and meta-analysis. Neurooncol Adv. 2020. 2: vdaa111