- Department of Neurosurgery, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Boston, Massachusetts United States.
- Department of Neurology, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Boston, Massachusetts United States.
- Department of Radiation Oncology, Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Boston, Massachusetts, United States.
- Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States.
Correspondence Address:
Ian F. Dunn
Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma, United States.
DOI:10.25259/SNI_487_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mark M. Zaki1, Saksham Gupta1, Blake Hauser1, Kyle C. Wu1, Robert M. Mallery2, Sashank Prasad2, Ayal Aizer3, Wenya Linda Bi1, Ian F. Dunn4. Bilateral occipital metastases: Visual deficits and management considerations. 11-Dec-2020;11:428
How to cite this URL: Mark M. Zaki1, Saksham Gupta1, Blake Hauser1, Kyle C. Wu1, Robert M. Mallery2, Sashank Prasad2, Ayal Aizer3, Wenya Linda Bi1, Ian F. Dunn4. Bilateral occipital metastases: Visual deficits and management considerations. 11-Dec-2020;11:428. Available from: https://surgicalneurologyint.com/surgicalint-articles/10448/
Abstract
Background: Metastases to the bilateral occipital lobes pose a difficult clinical scenario due to risk of debilitating visual loss. We sought to characterize clinical outcomes following different treatment modalities to help guide management in this challenging situation.
Methods: We retrospectively reviewed brain metastases patients treated at a single institution between 2008 and 2017 and assessed visual symptoms before and after treatment, the tumor and peritumoral edema volumes before treatment, and clinical outcomes including mortality.
Results: Eighteen patients with metastases affecting both occipital lobes were identified. Lung cancer represented the most common primary (n = 10). Visual deficits were present in 12 patients at the time of diagnosis of bilateral occipital metastases (67%). Patients received radiotherapy (n = 5) or combined surgical resection and radiotherapy (n = 13). Among symptomatic patients, two received radiation and 10 received combined surgery and radiation. Nine patients had improved visual symptoms after treatment with no new visual deficits reported as a result of treatment. Among asymptomatic patients, three were treated with radiation alone and three with resection and radiation. Three of these patients developed new visual symptoms following treatment, including one patient with Balint’s syndrome.
Conclusion: Patients with symptomatic bilateral occipital lobe metastases may experience visual improvement following intervention, especially if symptoms stem from compression or edema. Those without visual symptoms are at risk of developing new visual deficits during treatment, which should be included in the decision-making process and when counseling patients. Visual deficits improved after surgery in the majority of patients, with no cases of immediate visual deterioration.
Keywords: Blindness, Brain metastases, Occipital, Radiation, Vision
INTRODUCTION
Brain metastases are the most common intracranial tumors in adults, with a rising incidence as systemic disease control improves patient survival and screening increases with readily available imaging modalities.[
The nuanced impact of the treatment of brain metastases is highlighted in the scenario of metastases involving bilateral occipital lobes, which pose heightened risk to vision loss with or without intervention.[
MATERIALS AND METHODS
Data source and study design
We conducted a retrospective cohort study of brain metastases patients treated at a single institution from 2008 to 2017 to identify cases affecting both occipital lobes. We reviewed imaging, patient demographics, histology of the primary tumor, surgical details, visual symptoms, and volume of tumor and edema before and after therapy. The study design was reviewed and approved by the hospital’s Institutional Review Board (IRB). Patient consent was waived for retrospective chart review research, within the scope of IRB approval by our institution.
Inclusion and exclusion criteria
We included adult patients (age >18 years) diagnosed with bilateral occipital brain metastases (located between the occipital pole and parieto-occipital sulcus) who received radiation or surgical resection plus radiation treatment. We excluded patients if either occipital metastasis had a diameter under 1 cm at presentation to assess tumors for which surgery would be considered a treatment option.[
Statistical analysis
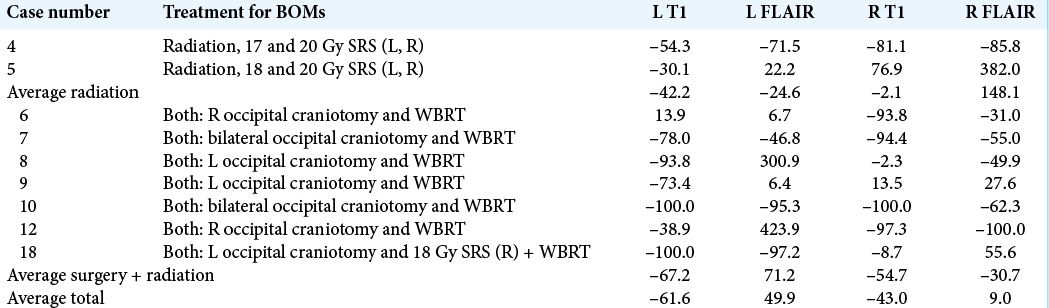
The volumes of enhancing tumor and associated peritumoral edema were independently segmented (Brainlab, Munich, Germany) for analysis before and following treatment using T1 postcontrast and T2 fluid attenuation inversion recovery (FLAIR) MRI sequences [
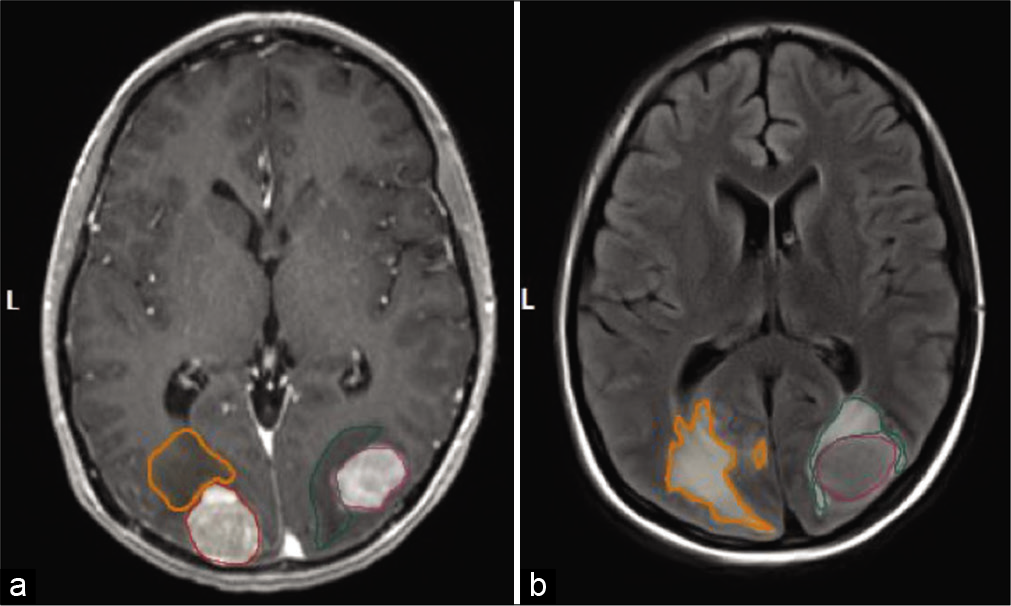
Figure 1:
Representative image of patient with bilateral occipital metastases highlighting tumor size and edema. (a) A representative T1 postgadolinium contrast signal of a patient is highlighted. Segmentation of the solid tumor was used for quantitative volumetric analysis. (b) A representative T2 FLAIR sequence signal of a patient is highlighted. Segmentation of surrounding edema was used for quantitative volumetric analysis. The outlined portions are as follows: red is right tumor volume, orange is R tumor edema, purple is left tumor volume, and green is left tumor edema.
RESULTS
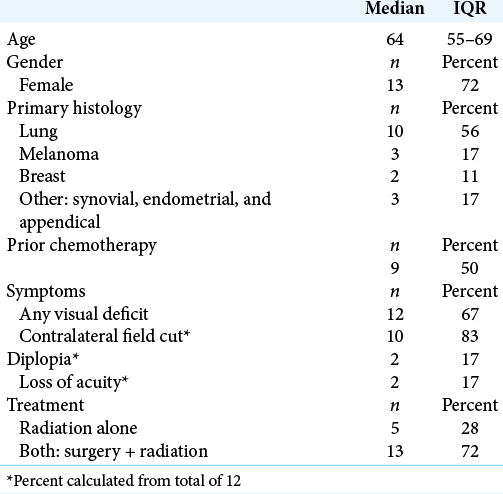
Patient characteristics
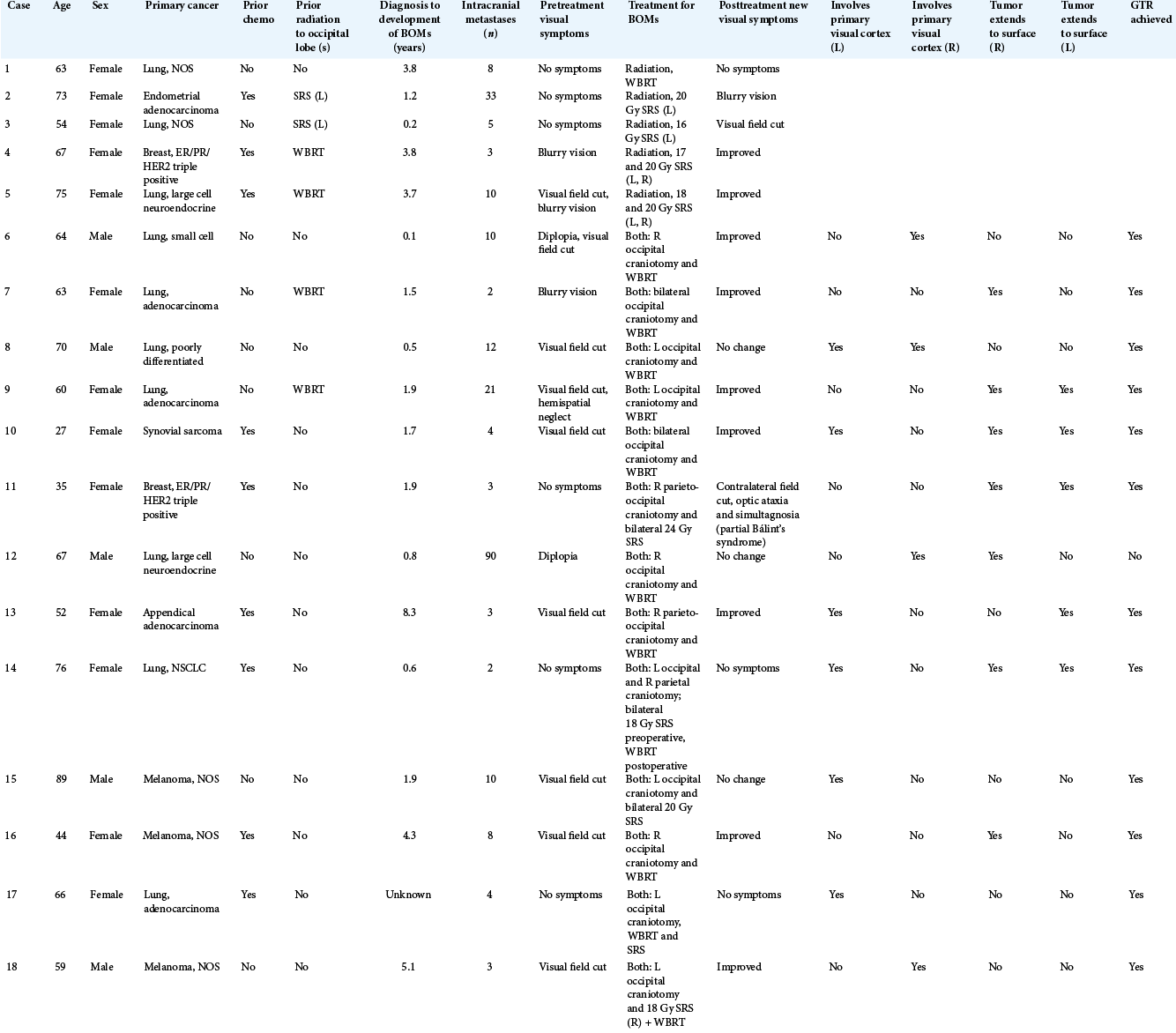
Eighteen patients (13 women and 5 men) with bilateral occipital metastases were identified [
Clinical presentation
Visual symptoms were present in 12 patients (67%) at time of presentation with bilateral occipital metastasis. These patients exhibited visual field deficit (67%), diplopia (17%), and visual acuity deficit (17%) on neurologic examination [
Visual symptoms before and after treatment
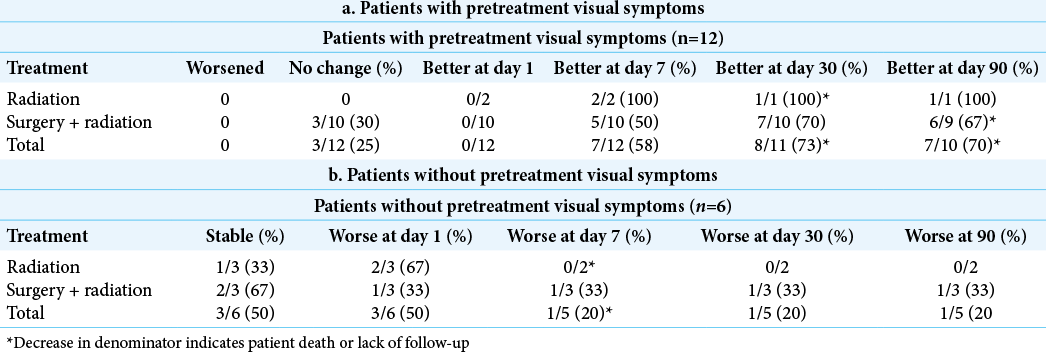
In patients with pretreatment visual deficits, no visual deficits worsened following treatment with either radiation or surgical resection plus radiation. Three patients remained with a stable deficit, whereas 9 of 12 patients improved. Of the three that did not improve, one patient had a biopsy rather than a gross total resection and presented with diplopia which was presumed secondary to a cavernous sinus metastasis [
In patients without pretreatment visual symptoms, half remained at baseline and half worsened throughout treatment. Of three patients who received radiation alone, one patient remained at baseline, one developed blurry vision which resolved within 1 week of treatment, and one developed a visual field cut within 1 day of treatment and subsequently died or was lost to follow up. Of three patients who received surgery plus radiation, two remained at baseline, whereas one patient developed a contralateral field cut, optic ataxia, and simultanagnosia (Balint’s syndrome) following 24 Gy of fractionated SRS. The patient then underwent surgical resection approximately 18 months later, and his visual deficits showed only miniscule improvement at 90 day follow-up [
Volumetric analysis
Nine of 18 patients had imaging pre- and postintervention available for volumetric analysis. Surgical resection showed greater reductions in postcontrast T1 volumes than radiation alone. Changes in FLAIR signal were variable across treatment modalities [
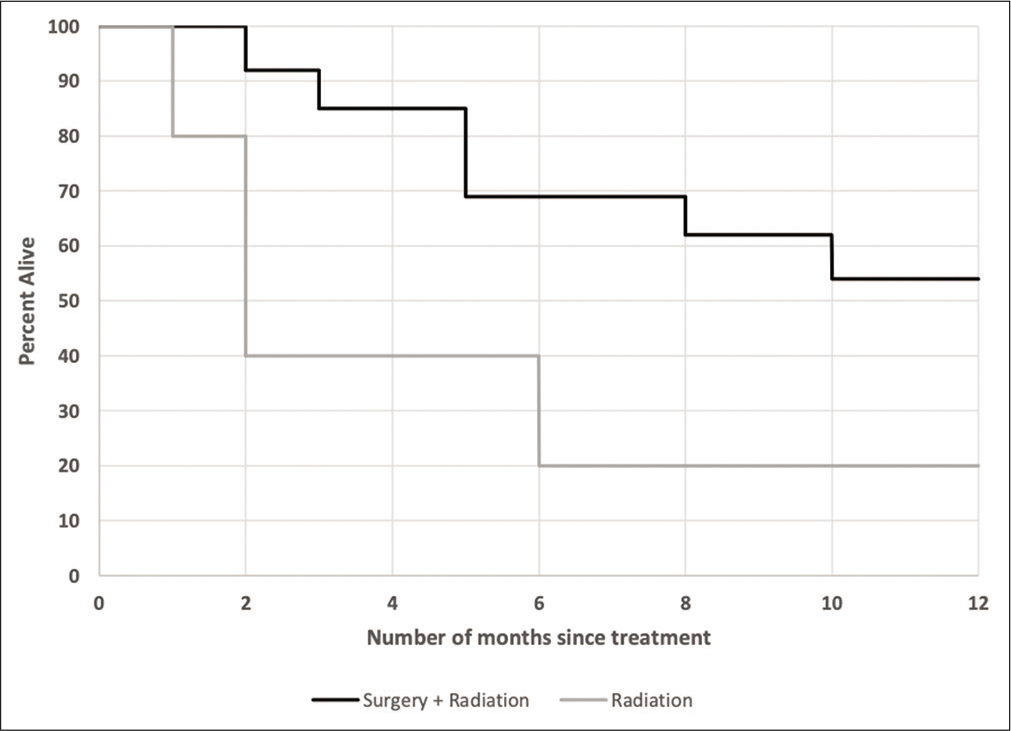
Mortality
Patients who underwent surgical selection plus radiation tended to have a higher likelihood of survival than those who underwent radiation alone at various follow-up times [
DISCUSSION
There is an absence of published experience in the management of patients with tumors involving bilateral occipital lobes.
A major risk of treating these patients is iatrogenic visual deficits, including cortical blindness, weighed against deficits conferred by the disease itself. In the setting of patients presenting with bilateral occipital metastases and unilateral visual field deficit, the decision to undergo bilateral treatment depends on extent of intracranial and extracranial tumor burden. In those with more severe tumor burden, unilateral treatment is favored to avoid unnecessary morbidity without a mortality benefit. When the bilateral occipital metastases are present in the setting of stable primary disease, treatment – especially surgical resection – may be safely conferred.
In our study, no patients who presented with visual deficits exhibited worsening of symptoms following treatment with any modality. Patients who presented with visual field deficit or blurry vision treated with surgical resection tended to gradually improve over days to weeks, likely due to relief of mass effect and reduction of edema.[
In patients without pretreatment visual deficits, the risk of iatrogenic visual deficits is an important consideration. In our cohort, one patient’s vision worsened months after radiation therapy which did not improve following surgical resection, and two patients’ vision worsened acutely following radiation alone. In terms of visual complications that may arise during treatment, one patient developed Bálint syndrome months following SRS. This may have been related to radiation, but could also have been tumor progression. Interestingly, the patient’s visual status did not improve following surgical resection.
Functional preservation is essential in patients with metastatic brain tumors to maximize quality of life. For patients who undergo surgical resection, meticulous care to preserve functional brain parenchyma, including selection of natural anatomic corridors to the surface of the tumor when subcortical, offers promising results in improving visual deficits in patients with bilateral occipital metastases. As subtotal resection is associated with decreased survival compared to a gross total resection, a gross total resection should be achieved when safely possible.[
Patients in our cohort who underwent surgical resection followed by radiation had markedly improved prognosis compared to radiation alone, which likely reflects selection bias in those patients deemed suitable for surgery. Our results appear consistent with literature in brain metastasis indicating improved survival benefit with surgical resection plus radiation compared to radiation alone.[
CONCLUSION
The management of bilateral occipital metastases involves consideration of symptomatology, disease burden, and goals of care. Patients often present with visual symptoms, which may improve following treatment, especially surgical resection. However, newly developed visual symptoms, including cortical blindness, are a potential consequence of treating these lesions. Surgical resection is a safe method for treatment in select patients with bilateral occipital metastases, especially if there is preoperative visual compromise, with careful consideration of anatomical corridors and brain parenchyma-sparing surgical technique.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L. Brain metastases. Nat Rev Dis Primers. 2019. 5: 5
2. Aldrich MS, Alessi AG, Beck RW, Gilman S. Cortical blindness: Etiology, diagnosis, and prognosis. Ann Neurol. 1987. 21: 149-58
3. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011. 22: 1-6
4. Lee CH, Kim DG, Kim JW, Han JH, Kim YH, Park CK. The role of surgical resection in the management of brain metastasis: A 17-year longitudinal study. Acta Neurochir (Wien). 2013. 155: 389-97
5. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012. 14: 48-54
6. Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014. 11: 203-22
7. Palmer JD, Trifiletti DM, Gondi V, Chan M, Minniti G, Rusthoven CG. Multidisciplinary patient-centered management of brain metastases and future directions. Neurooncol Adv. 2020. 2: vdaa034
8. Park YH, Kim TH, Jung SY, Kim YE, Bae JM, Kim YJ. Combined primary tumor and extracranial metastasis status as constituent factor of prognostic indices for predicting the overall survival in patients with brain metastases. J Korean Med Sci. 2013. 28: 205-12
9. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-500
10. Posner JB. Brain metastases: 1995. A brief review. J Neurooncol. 1996. 27: 287-93
11. Sharma M, Jia X, Ahluwalia M, Barnett GH, Vogelbaum MA, Chao ST. Cumulative intracranial tumor volume and number of brain metastasis as predictors of developing new lesions after stereotactic radiosurgery for brain metastasis. World Neurosurg. 2017. 106: 666-75
12. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012. 30: 419-25
13. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020. 17: 279-99
14. Team RC.editors. R: A Langage and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. p.
15. Yaeger KA, Nair MN. Surgery for brain metastases. Surg Neurol Int. 2013. 4: S203-8