- MD Program, Drexel University College of Medicine, Philadelphia,
- Department of Neurosurgery, Allegheny Health Network, Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Joshua Lucas, MD Program, Drexel University College of Medicine, Philadelphia, Pennsylvania, United States.
DOI:10.25259/SNI_1234_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Joshua Lucas1, Dorian Kusyk2, Donald Whiting2. Bilateral pallidal DBS for blepharospasm: A case report and review of the literature. 13-May-2022;13:200
How to cite this URL: Joshua Lucas1, Dorian Kusyk2, Donald Whiting2. Bilateral pallidal DBS for blepharospasm: A case report and review of the literature. 13-May-2022;13:200. Available from: https://surgicalneurologyint.com/surgicalint-articles/11590/
Abstract
Background: Deep brain stimulation (DBS) of the globus pallidus internus (GPi) in the treatment of craniocervical dystonia often requires an extended period of stimulation parameter manipulations.
Case Description: We present a patient suffering from debilitating blepharospasm treated with bilateral DBS of the GPi alongside 7 years of stimulation parameter manipulations and a literature review of comparable patients.
Conclusion: Our literature review suggests that a patient’s specific dystonic symptoms can guide stimulation parameter manipulations. Further research regarding trends in stimulation parameters being used in the field for different dystonic symptoms may expedite the stimulation parameter manipulation process.
Keywords: Blepharospasm, Deep brain stimulation, Dystonia, Globus pallidus internus
INTRODUCTION
There is a growing body of evidence regarding deep brain stimulation (DBS) of the globus pallidus internus (GPi) successfully treating dystonic symptoms and dystonic syndromes.[
We present a patient suffering from medically refractory craniofacial dystonia, primarily blepharospasm, who was successfully treated with bilateral DBS stimulation of the GPi. Our patient underwent a follow-up period of 7 years and 26 programming visits during which optimal stimulation parameters were gradually reached. We present this case alongside a comprehensive literature review of the various stimulation parameters currently used in DBS-treated blepharospasm. In doing so, our goal is to aide care providers in the stimulation parameter manipulation, alleviating both the patient and the care provider from some of the uncertainty embedded in this process. The patient in question gave us permission to share her clinical history, including programming parameters.
CASE DESCRIPTION
At the time of her DBS implantation, the patient was a 57-year-old Caucasian female who had presented with a several year history of debilitating, treatment resistant blepharospasm. Prior treatments with Botox® injections had not provided relief. After a discussion with the patient about the risks and benefits of surgical intervention, she was amenable to undergoing bilateral GPi DBS electrode placement. Planning and implantation was performed through our center’s standardized protocols, and the target was selected under direct visualization (17 mm lateral to the third ventricle wall, 5 mm below the AC PC on the left, and 3.5 mm below the AC PC).
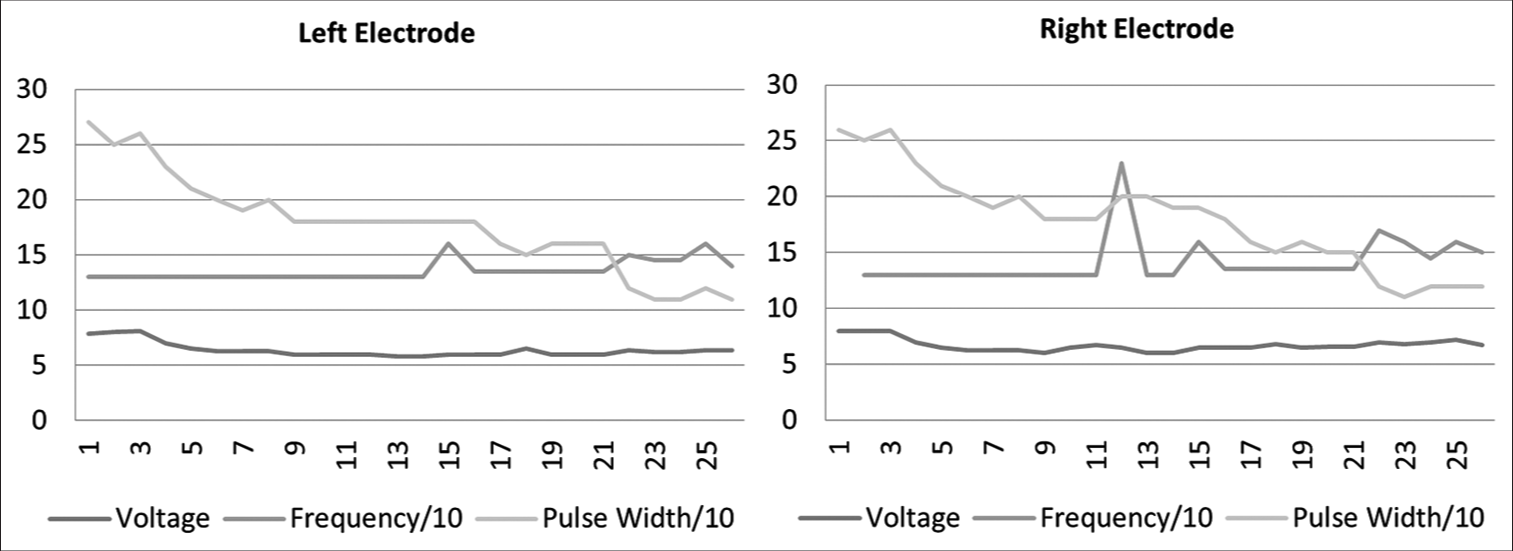
The patient was followed for 7 years at the time of writing this report. During this period, she had returned for stimulation parameter manipulation 26 times [
The first phase of her programming was targeted toward symptom control. Pulse width and voltage were gradually increased to help control her symptoms while minimizing side effects. This was achieved after about 1 year when she reported that it was “the best (I’ve) felt in a long time.” On sequential visits, there were attempts to decrease the charge density delivered to the patient by decreasing either the voltage, pulse width, or both without allowing for the re-emergence of her blepharospasm.
Throughout her follow-up, the patient also continued to receive Botox® injections and speech therapy. These synergistic interventions allowed for further decreases in delivered charge density.
DISCUSSION
Above we present a patient suffering from medically refractory craniofacial dystonia. While bilateral DBS stimulation of the GPi was effective, it included a follow-up period of 7 years encompassing 26 programming visits.
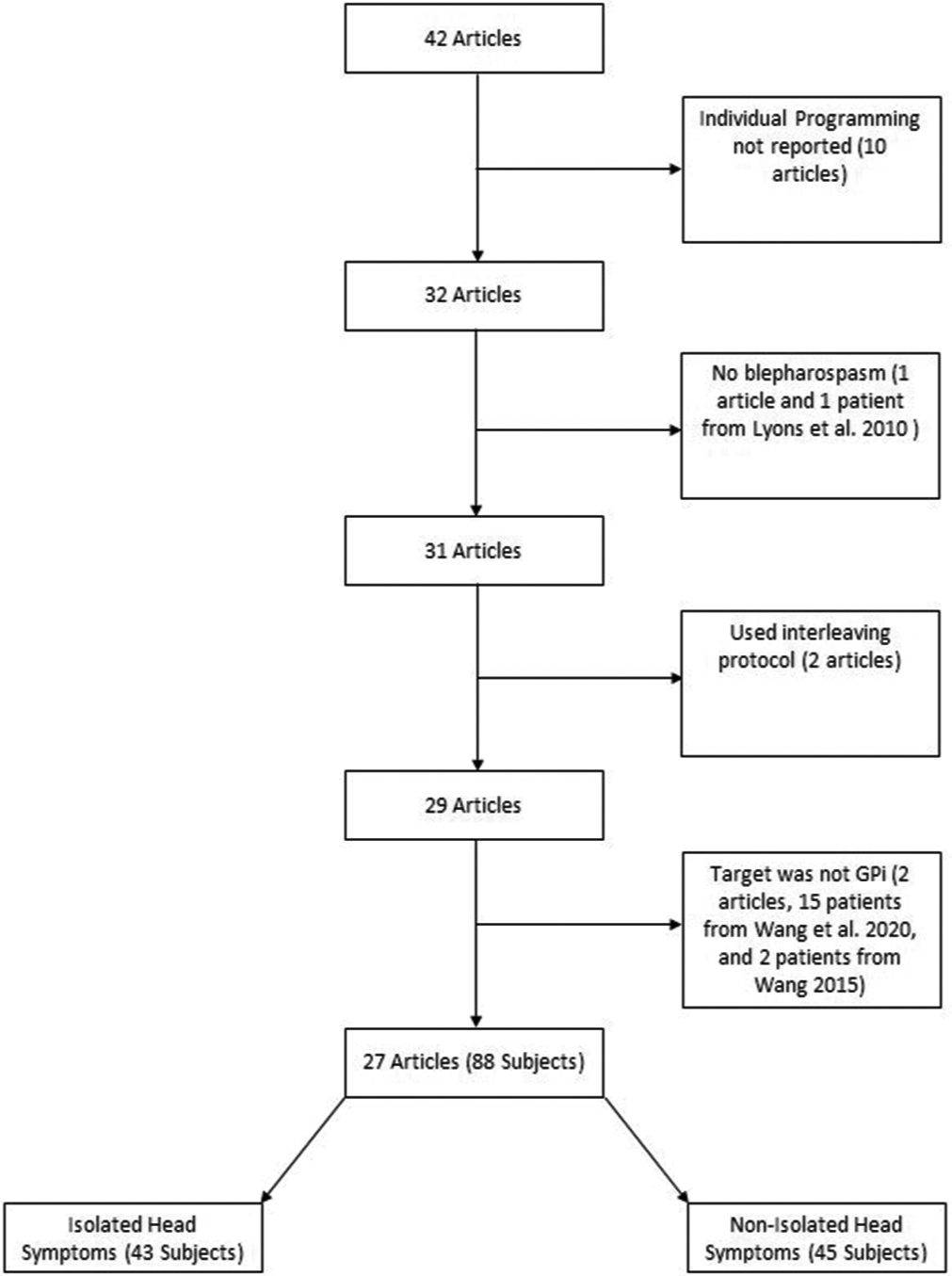
We conducted a PubMed literature review using the search terms “DBS AND Meige syndrome,” “DBS AND Blepharospasm,” and “DBS AND (Meige syndrome OR blepharospasm).” In total, 42 articles were included in our review, which included 381 patients. After applying exclusion criteria depicted in [
The average age of the 88 patients was 57 years old (range 26–73). There were 37 females, 48 males, and three instances in which sex was not reported. One patient received unilateral pallidal stimulation. Most patients suffered from more than 1 dystonic symptoms. The average stimulation parameters used in the patients in our literature review were a voltage of 3.2 V (range: 1–6.7), frequency of 146.1 Hz (10–235), and pulse width of 197.1 µs (60–500).
There exists evidence to suggest that elements of the basal ganglia, including the GPi, are organized somatotopically.[
Our patient’s final parameters consisted of considerably higher voltage than the literature reported; this may be due to the longer follow-up since the previous work has demonstrated a consistent decrease in electrode impedance and voltage increase over time.[
This brief analysis has some limitations. Although this case represents some of the longest follow-up published for patient with blepharospasm, it remains just one case. Furthermore, our analysis of the cases in the literature combines a variety of short interval time points along with inconsistently reported patient outcomes. Regardless, we believe that this case demonstrates that there needs to be more research on the long-term course of programming in DBS patients, as well as developing new programming protocols to decrease the time until symptom control.
CONCLUSION
DBS of the GPi has repeatedly proven its efficacy in providing patients with relief of their dystonic symptoms when other medical therapies fail. It is thus unfortunate that this surgical intervention must often be accompanied by an extended period of stimulation parameter manipulations, as was the case for the patient we presented in this report. In an effort to provide guidance to an under-studied aspect of this treatment option, we provide descriptive statistics of the various stimulation parameters currently being used in the field to treat a wide range of dystonic symptoms with preliminary evidence suggesting isolated cranial blepharospasm tends to have lower voltage requirements with longer pulse widths when compared to more globalized dystonic phenotypes.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aires A, Gomes T, Linhares P, Cunha F, Rosas MJ, Vaz R. The impact of deep brain stimulation on health related quality of life and disease-specific disability in Meige Syndrome (MS). Clin Neurol Neurosurg. 2018. 171: 53-7
2. Bae DW, Son BC, Kim JS. Globus pallidus interna deep brain stimulation in a patient with medically intractable meige syndrome. J Mov Disord. 2014. 7: 92-4
3. Bain PG, Liu X, Aziz TZ. Increase in the tactile catchment area of a sensory trick for alleviating blepharospasm following pallidal DBS. Mov Disord. 2009. 24: 624-6
4. Bally JF, Rohani M, Ruiz-Lopez M, Paramanandam V, Munhoz RP, Hodaie M. Patient-adjusted deep-brain stimulation programming is time saving in dystonia patients. J Neurol. 2019. 266: 2423-9
5. Bereznai B, Steude U, Seelos K, Bötzel K. Chronic high-frequency globus pallidus internus stimulation in different types of dystonia: A clinical, video, and MRI report of six patients presenting with segmental, cervical, and generalized dystonia. Mov Disord. 2002. 17: 138-44
6. Berman BD, Starr PA, Marks WJ, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg. 2009. 87: 37-44
7. Blomstedt P, Tisch S, Hariz MI. Pallidal deep brain stimulation in the treatment of Meige syndrome. Acta Neurol Scand. 2008. 118: 198-202
8. Capelle HH, Weigel R, Krauss JK. Bilateral pallidal stimulation for blepharospasm-oromandibular dystonia (Meige syndrome). Neurology. 2003. 60: 2017-8
9. Foote KD, Sanchez JC, Okun MS. Staged deep brain stimulation for refractory craniofacial dystonia with blepharospasm: Case report and physiology. Neurosurgery. 2005. 56: E415
10. Ghang JY, Lee MK, Jun SM, Ghang CG. Outcome of pallidal deep brain stimulation in meige syndrome. J Korean Neurosurg Soc. 2010. 48: 134-8
11. Hebb MO, Chiasson P, Lang AE, Brownstone RM, Mendez I. Sustained relief of dystonia following cessation of deep brain stimulation. Mov Disord. 2007. 22: 1958-62
12. Horisawa S, Ochiai T, Goto S, Nakajima T, Takeda N, Kawamata T. Long-term outcome of pallidal stimulation for Meige syndrome. J Neurosurg. 2018. 130: 84-9
13. Houser M, Waltz T. Meige syndrome and pallidal deep brain stimulation. Mov Disord. 2005. 20: 1203-5
14. Inoue N, Nagahiro S, Kaji R, Goto S. Long-term suppression of Meige syndrome after pallidal stimulation: A 10-year follow-up study. Mov Disord. 2010. 25: 1756-8
15. Kishore A, Panikar D, Balakrishnan S, Joseph S, Sarma S. Evidence of functional somatotopy in GPi from results of pallidotomy. Brain. 2000. 123: 2491-500
16. Limotai N, Go C, Oyama G, Hwynn N, Zesiewicz T, Foote K. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. J Neurol. 2011. 258: 2069-74
17. Loher TJ, Capelle HH, Kaelin-Lang A, Weber S, Weigel R, Burgunder JM. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008. 255: 881-4
18. Luthra NS, Mitchell KT, Volz MM, Tamir I, Starr PA, Ostrem JL. Intractable blepharospasm treated with bilateral pallidal deep brain stimulation. Tremor Other Hyperkinet Mov (N Y). 2017. 7: 472
19. Lyons MK, Birch BD, Hillman RA, Boucher OK, Evidente VG. Long-term follow-up of deep brain stimulation for Meige syndrome. Neurosurg Focus. 2010. 29: E5
20. Markaki E, Kefalopoulou Z, Georgiopoulos M, Paschali A, Constantoyannis C. Meige’s syndrome: A cranial dystonia treated with bilateral pallidal deep brain stimulation. Clin Neurol Neurosurg. 2010. 112: 344-6
21. Martinez-Torres I, Limousin P, Tisch S, Page R, Pinto A, Foltynie T. Early and marked benefit with GPi DBS for Lubag syndrome presenting with rapidly progressive life-threatening dystonia. Mov Disord. 2009. 24: 1710-2
22. Muta D, Goto S, Nishikawa S, Hamasaki T, Ushio Y, Inoue N. Bilateral pallidal stimulation for idiopathic segmental axial dystonia advanced from Meige syndrome refractory to bilateral thalamotomy. Mov Disord. 2001. 16: 774-7
23. Ostrem JL, Marks WJ, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranialcervical dystonia (Meige syndrome). Mov Disord. 2007. 22: 1885-91
24. Ouyang J, Hao Q, Zhu R, Wu G, Ding H, Wang D. Subthalamic Nucleus Deep Brain Stimulation in Primary Meige Syndrome: A 1-Year Follow-Up Study. Neuromodulation. 2021. 24: 293-9
25. Reese R, Gruber D, Schoenecker T, Bäzner H, Blahak C, Capelle HH. Long-term clinical outcome in meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. 2011. 26: 691-8
26. Romanelli P, Esposito V, Schaal DW, Heit G. Somatotopy in the basal ganglia: Experimental and clinical evidence for segregated sensorimotor channels. Brain Res Brain Res Rev. 2005. 48: 112-28
27. Romito LM, Elia AE, Franzini A, Bugiani O, Albanese A. Low-voltage bilateral pallidal stimulation for severe meige syndrome in a patient with primary segmental dystonia: Case report. Neurosurgery. 2010. 67: onsE308
28. Sako W, Morigaki R, Mizobuchi Y, Tsuzuki T, Ima H, Ushio Y. Bilateral pallidal deep brain stimulation in primary Meige syndrome. Parkinsonism Relat Disord. 2011. 17: 123-5
29. Santos AF, Veiga A, Augusto L, Vaz R, Rosas MJ, Volkmann J. Successful treatment of blepharospasm by pallidal neurostimulation. Mov Disord Clin Pract. 2016. 3: 409-11
30. Satzer D, Lanctin D, Eberly LE, Abosch A. Variation in deep brain stimulation electrode impedance over years following electrode implantation. Stereotact Funct Neurosurg. 2014. 92: 94-102
31. Sensi M, Cavallo MA, Quatrale R, Sarubbo S, Biguzzi S, Lettieri C. Pallidal stimulation for segmental dystonia: long term follow up of 11 consecutive patients. Mov Disord. 2009. 24: 1829-35
32. Shu W, Li Y, Li J, Zhang Y. Interleaving programming in pallidal deep brain stimulation improves outcomes in a patient with Meige syndrome. Br J Neurosurg. 2018. 32: 661-2
33. Sobstyl M, Brzuszkiewicz-Kuźmicka G, Zaczyński A, Pasterski T, Aleksandrowicz M, Ząbek M. Long-term clinical outcome of bilateral pallidal stimulation for intractable craniocervical dystonia (Meige syndrome) Report of 6 patients. J Neurol Sci. 2017. 383: 153-7
34. Tai CH, Wu RM, Liu HM, Tsai CW, Tseng SH. Meige syndrome relieved by bilateral pallidal stimulation with cycling mode: Case report. Neurosurgery. 2011. 69: E1333-7
35. Tian H, Yu Y, Zhen X, Zhang L, Yuan Y, Zhang B. Long-term efficacy of deep brain stimulation of bilateral globus pallidus internus in primary meige syndrome. Stereotact Funct Neurosurg. 2019. 97: 356-61
36. Vagefi MR, Lin CC, McCann JD, Anderson RL. Exacerbation of blepharospasm associated with craniocervical dystonia after placement of bilateral globus pallidus internus deep brain stimulator. Mov Disord. 2008. 23: 454-6
37. Vercueil L, Pollak P, Fraix V, Caputo E, Moro E, Benazzouz A. Deep brain stimulation in the treatment of severe dystonia. J Neurol. 2001. 248: 695-700
38. Waln O, Ledoux MS. Blepharospasm plus cervical dystonia with predominant anterocollis: A distinctive subphenotype of segmental craniocervical dystonia?. Tremor Other Hyperkinet Mov (N Y). 2011. 2011: 33
39. Wang X, Mao Z, Cui Z, Xu X, Pan L, Liang S. Predictive factors for long-term clinical outcomes of deep brain stimulation in the treatment of primary Meige syndrome. J Neurosurg. 2019. 132: 1367-75
40. Wang X, Zhang C, Wang Y, Liu C, Zhao B, Zhang JG. Deep brain stimulation for craniocervical dystonia (Meige syndrome): A report of four patients and a literature-based analysis of its treatment effects. Neuromodulation. 2016. 19: 818-23
41. Wang X, Zhang Z, Mao Z, Yu X. Deep brain stimulation for Meige syndrome: A meta-analysis with individual patient data. J Neurol. 2019. 266: 2646-56
42. Woehrle JC, Blahak C, Kekelia K, Capelle HH, Baezner H, Grips E. Chronic deep brain stimulation for segmental dystonia. Stereotact Funct Neurosurg. 2009. 87: 379-84
43. Yamada K, Shinojima N, Hamasaki T, Kuratsu J. Pallidal stimulation for medically intractable blepharospasm. BMJ Case Rep. 2016. 2016: bcr2015214241
44. Yao C, Horn A, Li N, Lu Y, Fu Z, Wang N. Post-operative electrode location and clinical efficacy of subthalamic nucleus deep brain stimulation in Meige syndrome. Parkinsonism Relat Disord. 2019. 58: 40-5
45. Zauber SE, Watson N, Comella CL, Bakay RAE, Metman LV. Stimulation-induced parkinsonism after posteroventral deep brain stimulation of the globus pallidus internus for craniocervical dystonia: Case report. J Neurosurg. 2009. 110: 229-33
46. Zhan S, Sun F, Pan Y, Liu W, Huang P, Cao C. Bilateral deep brain stimulation of the subthalamic nucleus in primary Meige syndrome. J Neurosurg. 2018. 128: 897-902