- Department of Head and Neck Surgery, Neurosurgery Unit, Garibaldi Hospital, Catania, Italy
- Neurosurgical Clinic AOUP “Paolo Giaccone”, Post Graduate Residency Program in Neurologic Surgery, Department of Biomedicine Neurosciences and Advanced Diagnostics, School of Medicine, University of Palermo, Palermo, Italy
- Department of Radiology, Garibaldi Hospital, Catania, Italy.
- Department of Neurosurgery, Trauma Center, Gamma knife Center, Cannizzaro Hospital, Catania, Italy.
Correspondence Address:
Gianluca Scalia, Department of Head and Neck Surgery, Neurosurgery Unit, Garibaldi Hospital, Catania, Italy.
DOI:10.25259/SNI_1056_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gianluca Scalia1, Salvatore Marrone2, Roberta Costanzo2, Massimiliano Porzio2, Carmelo Riolo1, Massimiliano Giuffrida1, Giancarlo Ponzo1, Giuseppe Vasta1, Massimo Furnari1, Domenico Gerardo Iacopino2, Giovanni Federico Nicoletti1, Francesca Graziano1, Gianluca Galvano3, Giuseppe Emmanuele Umana4. Bilateral post-traumatic hygromas in patient with frontotemporal dementia. 30-Dec-2022;13:597
How to cite this URL: Gianluca Scalia1, Salvatore Marrone2, Roberta Costanzo2, Massimiliano Porzio2, Carmelo Riolo1, Massimiliano Giuffrida1, Giancarlo Ponzo1, Giuseppe Vasta1, Massimo Furnari1, Domenico Gerardo Iacopino2, Giovanni Federico Nicoletti1, Francesca Graziano1, Gianluca Galvano3, Giuseppe Emmanuele Umana4. Bilateral post-traumatic hygromas in patient with frontotemporal dementia. 30-Dec-2022;13:597. Available from: https://surgicalneurologyint.com/surgicalint-articles/12086/
Abstract
Background: Frontotemporal dementia (FTD) is a highly disabling neurologic disorder characterized by behavioral alterations and movement disorders, involving patients with a mean age of 58 years. We present a unique case of a patient suffering from FTD who developed post traumatic bilateral hygromas.
Case Description: A 52-year-old male patient, with an history of head trauma 3 months before, was admitted to our department for recurrent motor seizures. Anamnesis was positive for FTD with severe frontal syndrome. Brain computed tomography and magnetic resonance imaging (MRI) showed the typical “knife-blade” appearance of the cortical atrophy associated to bilateral hemispheric hygromas exerting mild mass effect. Brain MRI showed the signs of the cortical and “anti-cortical” vein. The two subdural collections were evacuated through two bilateral burr holes and controlled drainage. Despite anti-epileptic drugs therapy, in the early postoperative period, the patient presented further tonic-clonic seizures. The patient showed progressive recovery and was transferred to the neurorehabilitation center. After 6-month follow-up, he completely recovered.
Conclusion: In FTD, severe cortical atrophy leads to space increase between arachnoid and pia mater that could affect the anatomical integrity especially after trauma, with possible development of hygromas. The coexistence of radiological findings of the cortical vein and sign of the “anti-cortical” vein can make difficult an exact differential diagnosis between a primitive hygroma and a Virchow hygroma from resorption of previous blood collection. Surgical treatment may be indicated in selected patients, but it is burdened by higher postoperative risks compared to the general population.
Keywords: Cortical veins, Dementia, Hygromas, Seizures, Subarachnoid space
INTRODUCTION
Frontotemporal dementia (FTD) is a severe dementia involving patients in the fourth–fifth decade, with a mean age of onset at 48 years old and with a prevalence, regardless of gender, of about 15/100.000 individuals. It is a highly disabling disease with a prognosis of 3–10 years from the onset. Exitus is mainly associated with causes secondary to frontal involvement causing progressive limitation of movements till forced bed stay which may lead to pneumonia, cardiovascular, and respiratory failure. Clinically, it is possible to find signs and symptoms related to frontal (behavioral alterations and movement disorders) and temporal atrophy (speech disorders with mixed aphasia and memory alterations). Magnetic resonance imaging (MRI) and PET allow to document the severity of cortical atrophy. We present an adult patient presenting the unique association of FTD and bilateral hygromas that rapidly worsened the symptoms related to FTD.
CASE ILLUSTRATION
History and physical examination
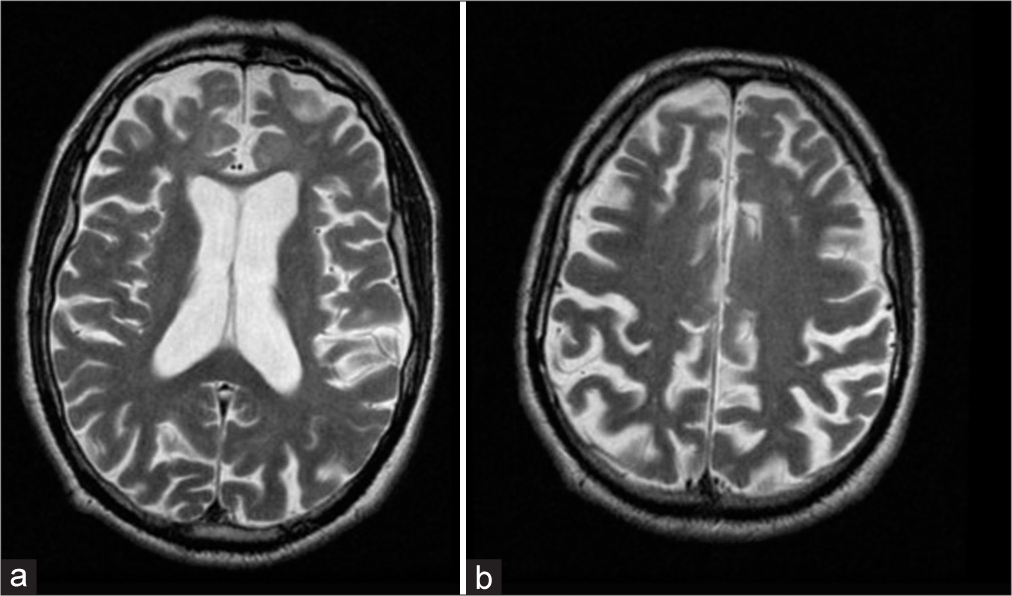
A 52-year-old male patient suffering from FTD with severe frontal syndrome (kleptomania, restlessness, and impulsivity), alteration of attention and memory, semantic aphasia, echolalia, and bladder and bowel incontinence was admitted at our department for recurrent motor seizures. Three months earlier, he was referred to the emergency department for a head trauma related to a seizure episode. He had performed brain MRI after trauma, which showed no acute relevant signs [
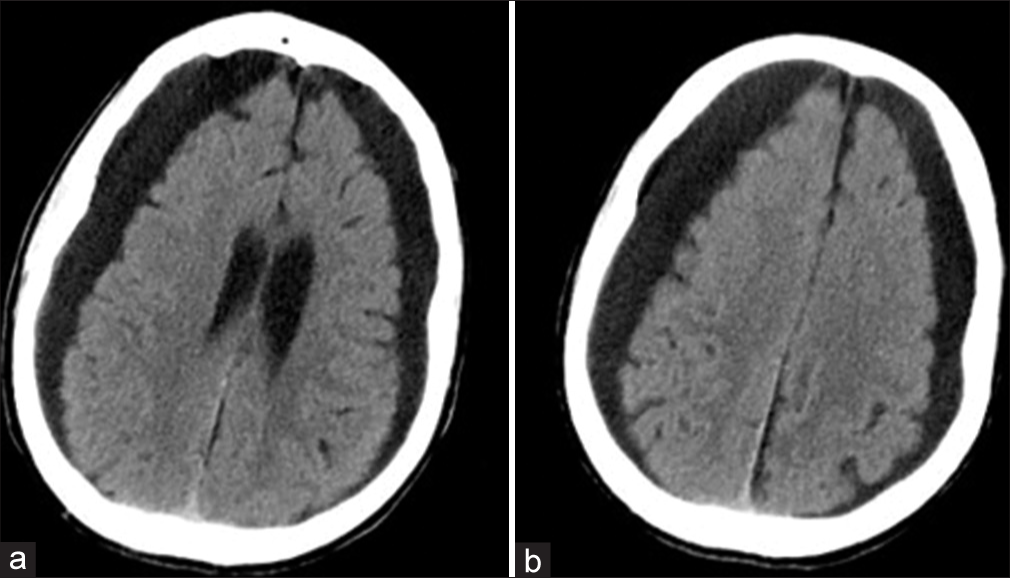
Brain computed tomography (CT) showed the typical “knife-blade” appearance of the cortical atrophy associated to bilateral hemispheric hygromas 20 mm thick and exerting mild mass effect [
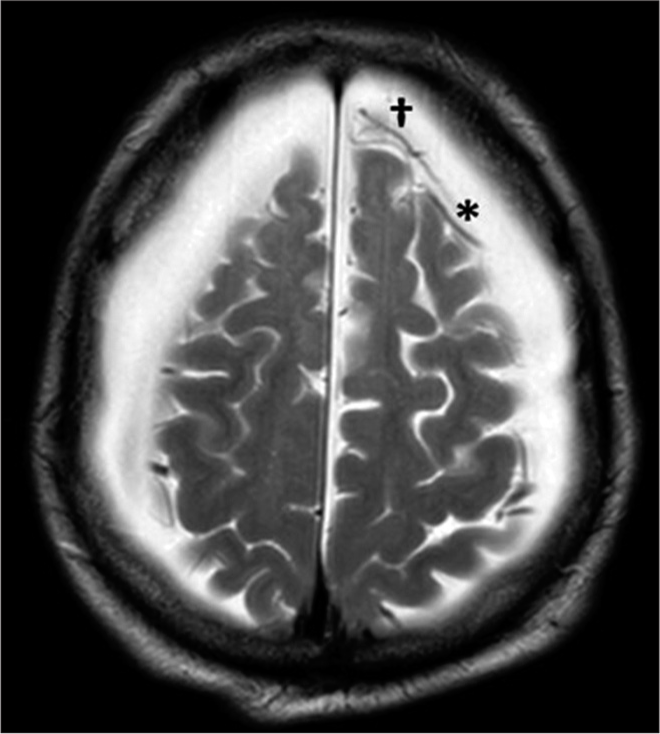
Axial T1-weighted gadolinium-enhanced MRI sequences showed the signs of the cortical vein and “anti-cortical” vein: the first is typical of subdural fluid collections, the latter indicates sub-arachnoid space enlargement [
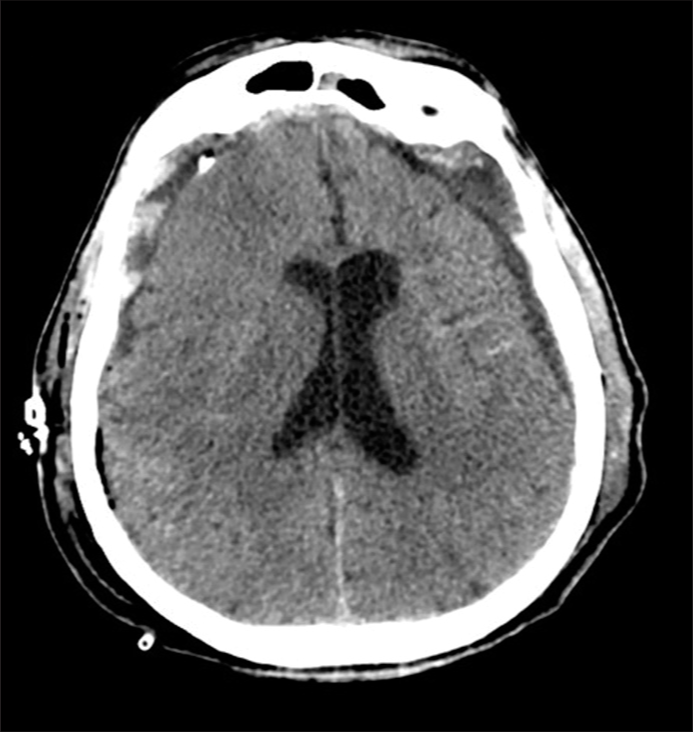
The patient underwent two bilateral parietal burr holes as well as slow controlled drainage of the subdural collections. Unfortunately, in the early postoperative period, the patient presented further tonic-clonic seizures, despite the treatment with AEDs. An immediate postoperative brain CT scan showed a satisfying drainage of the subdural collections and a thin sylvian subarachnoid hemorrhage [
DISCUSSION
FTD
Cortical atrophic degeneration is a progressive and irreversible disease that slowly reduces the functionality of the frontal and temporal lobes as confirmed by the CT or MRI imaging and by reduced accumulation of the 18-FDG of PET study.[
Pathophysiology
Although the phenotype is predominantly sporadic, in 30% of cases, there may be a characteristic genotypic predisposition (e.g., MAPT tau-gene in FTLD-tau). Characteristic findings of this disease are silvery intracytoplasmic inclusions, called Pick bodies, present in atrophic areas and straight filamentous intraneural aggregates of tau protein <30 millimicrons in size.
Clinical presentation and management
Clinical features include signs and symptoms related to frontal and temporal atrophy. The most frequent and usually early diagnosed is the frontal syndrome characterized by behavioral alterations, apathy, movement disorders (pyramidal and extrapyramidal), and disinhibition. In the temporal syndrome, on the other hand, there are speech disorders with mixed aphasia, depending on the hemisphere involved, and memory, at least at the beginning, episodic memory (unlike Alzheimer’s disease).[
FTD and hygromas
The severe atrophy that takes place approximately symmetrically in both cortexes inevitably leads to an increase in the subarachnoid space that is associated with a greater tension of bringing veins and arachnoid trabecula, and to a possible excessive distention of the thinnest layer of this membrane that is most vulnerable to continuous stresses. From this comes the risk of development of micro-ruptures with the formation of breaches which enlarges overtime causing CSF leakage from the subarachnoid space at higher pressure to the subdural space at lower pressure. This would justify the development of bilateral hygromas which may progressively increase with gradual enlargement of the fluid collection and can contribute to symptom’s worsening, as described in our case.
Primitive subdural hygroma is defined as a fluid collection in the subdural space secondary to post-traumatic lacerations of the arachnoid membrane and passage of CSF into the subdural space which differs from subdural hematoma due to its remarkable albuminic content and the absence of the two typical membranes (parietal and visceral). The degree of cerebral atrophy along with cranial morphology would affect (pulling force) on arachnoid trabecula. Brain atrophy helps to increase the virtual space between the inner cranial table and pia mater producing intracranial negative pressure, constant at each site (Boyle law). When the distance is greater than the length of the trabecula, the risk of stratification of the inner layer of the dura mater increases, the subdural space becomes real and sufficient to be filled with fluids (blood and liquor)[
There are few cases of bilateral hygromas described in the literature, nevertheless none of these is related to severe frontotemporal cortex bilateral atrophy. Bilateral hygromas are often found in spontaneous intracranial hypotension (SIH),[
The coexistence of radiological signs typical of a collection between dura and arachnoid (sign of the cortical vein and typical in hygromas) and of an increase in the space between arachnoid and cerebral cortex (the “anti-cortical” vein sign and typical of grey matter atrophy) was in the present case highlighted.[
Based on the hypothesis that voluminous hygromas may cause irritative phenomena on the cortex,[
Finally, postsurgical epilepsy with cranial shaking may lead to excessive traction and rupture of some bridging veins (already under tension and stretching due to such an important cortical atrophy), favoring blood diffusion to the subarachnoid space, through a previous arachnoid breach, irritating the cortex and self-maintaining epileptic events. The surgical procedure should be performed simultaneously and slowly with mini-craniectomies or through minimally invasive technique[
CONCLUSION
In FTD, severe cortical atrophy leads to an increase in the space between arachnoid and pia mater that could affect the anatomical integrity of the first membrane especially after trauma, with possible development of hygromas. The coexistence of radiological findings of the cortical vein and sign of the “anti-cortical” vein can make difficult making exact differential diagnosis between a primitive hygroma and a Virchow hygroma from resorption of the previous blood collection. The evacuation of the collection must be careful, slow, and controlled. In consideration of the high risks of complications due to the modification of precarious balances in fragile patient population, a careful surgical indication is warranted and paucisympomatic patients should be excluded from the study.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Cohen-Gadol AA. Remote contralateral intraparenchymal hemorrhage after overdrainage of a chronic subdural hematoma. Int J Surg Case Rep. 2013. 4: 834-6
2. Davidson B, Nassiri F, Mansouri A, Badhiwala JH, Witiw CD, Shamji MF. Spontaneous intracranial hypotension: A review and introduction of an algorithm for management. World Neurosurg. 2017. 101: 343-9
3. Doval O, Gaviria M. Demencia frontotemporal [Fronto-temporal dementia]. Actas Esp Psiquiatr. 2000. 28: 13-28
4. Fischer M, Schmutzhard E. Das posteriore reversible Enzephalopathiesyndrom [Posterior reversible encephalopathy syndrome]. Med Klin Intensivmed Notfmed. 2016. 111: 417-24
5. Galimberti D, Fumagalli GG, Fenoglio C, Cioffi SM, Arighi A, Serpente M. Progranulin plasma levels predict the presence of GRN mutations in asymptomatic subjects and do not correlate with brain atrophy: Results from the GENFI study. Neurobiol Aging. 2018. 62: 245.e9-245.e12
6. Gilmour GS, Scott J, Couillard P. Leaking the diagnosis: A case of convulsive status epilepticus due to intracranial hypotension. Neurocrit Care. 2019. 31: 562-6
7. Jang UJ, Lee KS, Shim JJ, Yoon SM, Doh JW. Diagnostic value of the cortical vein sign: Unreliable index of atrophy on MR image. J Korean Neurotraumatol Soc. 2006. 2: 13-7
8. Kim CH, Song GS, Kim YH, Kim YS, Sung SK, Son DW. Remote hemorrhage after burr hole drainage of chronic subdural hematoma. Korean J Neurotrauma. 2017. 13: 144-8
9. Kim JH, Roh H, Yoon WK, Kwon TH, Chong K, Hwang SY. Clinical features of patients with spontaneous intracranial hypotension complicated with bilateral subdural fluid collections. Headache. 2019. 59: 775-86
10. Lee KS. Chronic subdural hematoma in the aged, trauma or degeneration?. J Korean Neurosurg Soc. 2016. 59: 1-5
11. Li W, Xiao WM, Luo GP, Liu YL, Qu JF, Fang XW. Asymmetrical cortical vein sign predicts early neurological deterioration in acute ischemic stroke patients with severe intracranial arterial stenosis or occlusion. BMC Neurol. 2020. 20: 331
12. McCluney KW, Yeakley JW, Fenstermacher MJ, Baird SH, Bonmati CM. Subdural hygroma versus atrophy on MR brain scans: “The cortical vein sign”. AJNR Am J Neuroradiol. 1992. 13: 1335-9
13. Mokri B. Spontaneous intracranial hypotension. Curr Neurol Neurosci Rep. 2001. 1: 109-17
14. Neary D, Snowden J. Fronto-temporal dementia: Nosology, neuropsychology, and neuropathology. Brain Cogn. 1996. 31: 176-87
15. Ogasawara K, Ogawa A, Okuguchi T, Kobayashi M, Suzuki M, Yoshimoto T. Postoperative hyperperfusion syndrome in elderly patients with chronic subdural hematoma. Surg Neurol. 2000. 54: 155-9
16. Shields LB, Johnson JR, Shields CB. Posterior reversible encephalopathy syndrome following a thoracic discectomy-induced dural leak: Case report. J Neurosurg Spine. 2016. 25: 586-90
17. Srivastava C, Sahoo SK, Ojha BK, Chandra A, Singh SK. Subdural hygroma following endoscopic third ventriculostomy: Understanding the pathophysiology. World Neurosurg. 2018. 118: e639-45
18. Sun W, Liu W, Zhang Z, Xiao L, Duan Z, Liu D. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: A novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur J Neurol. 2014. 21: 1411-8
19. Uchida D, Fujimoto A, Yamazoe T, Yamamoto T, Enoki H. Seizure frequency can be reduced by changing intracranial pressure: A case report in drug-resistant epilepsy. Epilepsy Behav Case Rep. 2018. 10: 14-7
20. Umana GE, Chiriatti S, Fricia M, Alberio N, Cicero S, Nicoletti GF, editors. Letter to the editor regarding “Twist drill procedure for chronic subdural hematoma evacuation-an analysis of predictors for treatment success”. World Neurosurg. 2020. 139: 698
21. Umana GE, Chiriatti S, Roca E, Scalia G, Fricia M, Alberio N. New tools in percutaneous minimally invasive chronic subdural hematomas evacuation. Interdiscip Neurosurg. 2020. 21: 100736
22. Umana GE, Raudino G, Alberio N, Inserra F, Giovinazzo G, Fricia M. Slit-like hypertensive hydrocephalus: Report of a late, complex, and multifactorial complication in an oncologic patient. Surg Neurol Int. 2020. 11: 219