- Department of Neurosurgery, Institute of Psychiatry and Neurology, Warsaw, Poland
Correspondence Address:
Marek Prokopienko, MD, PhD, Department of Neurosurgery, Institute of Psychiatry and Neurology, Warsaw, Poland.
DOI:10.25259/SNI_821_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Marek Prokopienko, Michał Sobstyl. Biological and hardware-related spinal cord stimulation complications and their management: A single-center retrospective analysis of the implantation of nonrechargeable implantable pulse generators in different pain conditions. 08-Nov-2024;15:402
How to cite this URL: Marek Prokopienko, Michał Sobstyl. Biological and hardware-related spinal cord stimulation complications and their management: A single-center retrospective analysis of the implantation of nonrechargeable implantable pulse generators in different pain conditions. 08-Nov-2024;15:402. Available from: https://surgicalneurologyint.com/surgicalint-articles/13213/
Abstract
Background: We present our experience with spinal cord stimulation (SCS) for patients suffering from different pain conditions who subsequently developed hardware-related complications after SCS surgery. The SCS hardware-related complications may compromise the continuous SCS therapy due to partial or total hardware removal. Such situations should be avoided, and possible predisposing factors for their development should be minimized. The present study aimed to evaluate the frequency of hardware-related complications and their proper neurosurgical management.
Methods: The study is designed as a retrospective analysis of all hardware-related complications of SCS procedures for pain patients who underwent the implantation of the nonrechargeable PrimeAdvanced™ SureScan™ magnetic resonance imaging (MRI) neurostimulator (Medtronic, Minneapolis, United States). This neurostimulator allows patients safe access to MRI scans anywhere on the body. The PrimeAdvanced™ SureScan™ MRI neurostimulator can deliver stimulation through one or more leads in the epidural space. From December 2017 to December 2021, 20 patients with SCS implantations and a minimum postoperative follow-up of 3 months were included. All patients were operated on using identical surgical and intraprocedural techniques. The same SCS hardware was implanted (nonrechargeable PrimeAdvanced™ SureScan™ MRI neurostimulator) in all patients. We examined numerous preoperative variables (i.e., sex, age at surgery, diabetes, body mass index, and type of pain syndrome) to detect any correlation between them and the incidence of postoperative hardware-related complications.
Results: Among 20 patients, 8 (40%) patients were affected by hardware-related complications. The most common complications were skin erosion found in 5 patients (25%) and incorrect functioning of the implantable pulse generator (IPG) affecting 2 patients (10%). There were 1 case of an IPG migration (5%) and 1 hardware infection (5%) due to a staphylococcal wound. A total number of 16 revision surgeries were performed to manage all hardware-related complications in these patients adequately. Most of the patients (5 of them) were troubled by more than one hardware-related complication episode. Three patients had 3 revision surgeries, 2 patients had 2 revision surgeries, and 3 patients had 1 revision surgery. Among 8 patients with complications, 3 patients had no further continuation of SCS therapy due to hardware-related complications. Among these 3 patients who stopped their SCS therapy, 1 patient had 3 hardware-related episodes, and the remaining 2 patients were troubled by two hardware-related episodes before discontinuation of SCS therapy.
Conclusion: Our results indicate that patients treated by the SCS technique are at higher risk for the development of skin-related complications, especially skin erosions and less common skin infections, notably in cases when large (high profile) IPGs are utilized. The use of smaller IPGs could reduce the number of these biological as well as hardware-related complications and associated revision surgeries.
Keywords: Complex regional pain syndrome, Failed back surgery syndrome, Hardware-related complications, Skin erosion, Spinal cord stimulation, Visual Analog Scale
INTRODUCTION
The basis for spinal cord stimulation (SCS) has its origin in the gate control theory of pain proposed in 1965 by Melzack and Wall.[
The main indications for SCS implantations are: failed back surgery syndrome (FBSS) and CRPS.[
This study aimed to determine the frequency of hardware complications and to determine the factors predisposing their development.
MATERIALS AND METHODS
We performed a retrospective evaluation of the clinical outcomes in 20 consecutively treated patients (9 patients with severe, intractable CRPS and 11 patients with FBSS). All patients underwent implantation of a nonrechargeable PrimeAdvanced™ SureScan™ magnetic resonance imaging (MRI) neurostimulator (Medtronic Inc. Minneapolis, MN, USA) between December 2017 and December 2021.
Due to the retrospective analysis of the presented clinical data, the institutional approval by the Ethics Committee has been waived. All patients were informed about possible complications related to SCS surgery and provided signed, written informed consent before SCS treatment. All patients selected for this study had previously received conventional pharmacological treatment, including multi-modal pain therapy based on multiple pharmacological blockades. All patients were referred for SCS treatment by an experienced pain specialist or a specialized pain center.
Only 16-electrode Specify™ SureScan™ MRI surgical paddle-style SCS leads (Medtronic Inc. Minneapolis, MN, USA) were used. Implantations were performed using the same surgical procedure. SCS treatment was performed in two stages. The same surgical technique was used for all patients. SCS electrodes were implanted under general anesthesia with fluoroscopic guidance for final SCS lead placement in the spinal epidural space. Opening of the spinal canal was achieved by removing the supraspinous, interspinal, and flavum ligaments. Vertebral laminae were not removed. This surgical maneuver allowed for a significant reduction in venous bleeding from spinal bone structures.
On the 1st day following surgery, a stimulation screening was performed to cover the painful area with an acceptable level of SCS-induced paresthesia. During screening, the SCS electrodes were connected to the external stimulator provided by the Medtronic manufacturer. Patients were usually discharged on the 2nd or 3rd postoperative day. Over the next 2 weeks, if the patients showed significant benefit, i.e., at least 50% pain reduction assessed using the Visual Analog Scale (VAS), we proceeded with the implantation of the Prime Advanced Sure Scan™ nonrechargeable implantable pulse generator (IPG) (Medtronic Inc. Minneapolis, MN, USA). Implantation was performed under local anesthesia and with intravenous sedation. The illustration of the final placement of the connected implantable pulse generator, usually in the right buttock area, is depicted in
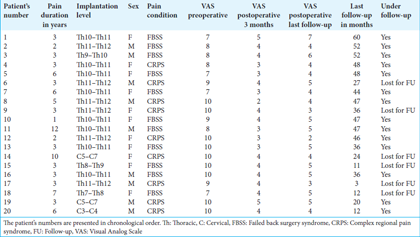
The patient cohort was comprised of 11 females and 9 males. The mean age of initial diagnosis was 42 years (range: 25–77 years), and the mean duration of disease before SCS treatment was 4 years (range: 1–12 years). FBSS was diagnosed in 11 patients (55%), and CRPS was diagnosed in 9 patients (45%). In 17 patients (85%), the electrode was placed in the thoracic segment in 3 cases (15%) in the cervical region.
The pain intensity was assessed using VAS scores at baseline, 3 months, and in long-term follow-up in individual patients. A detailed neuropsychological examination was also performed on these patients. For this article, the results of the neuropsychological examination were presented only in relation to the Addenbrooke’s Cognitive Examination-III (ACE-III) cognitive function screening scale. The ACE-III cognitive function scores were assessed at baseline, at 3-month follow-up, and the last available follow-up.
Descriptive statistics were applied to all measures, and numerical data were expressed as mean and interquartile ranges. The pain intensity using VAS scores was analyzed using a t-test.
RESULTS
Among 24 patients screened and suitable for two-staged SCS therapy, 4 patients failed to respond positively to the SCS 2-weeks trial period. All 4 patients suffered from FBSS. Four patients with the diagnosis of FBSS had their SCS electrodes removed with uncomplicated further follow-up. In contrast, all 9 patients with a preoperative diagnosis of CRPS responded favorably to the 2-week duration stimulation trial period. In 19 cases (96%), the IPG was placed in the buttock area, while in the remaining 1 patient, the IPG was placed in the abdominal wall.
The results of clinical characteristics and individual VAS scores in chronological order in consecutive patients are shown in
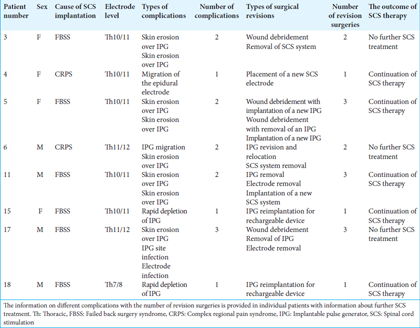
In this study, 8 patients (40%) developed postoperative complications. The most common complications in our series were so-called biological complications. The skin erosions were most frequently encountered, which were undoubtedly related to the large dimensions of the nonrechargeable PrimeAdvanced™ SureScan™ MRI neurostimulator. The typical skin erosion and wound dehiscence over IPG are shown in
The other hardware-related complications included 2 cases of fast depletion of IPG found in 2 patients with FBSS who required relatively high stimulation settings to provide excellent pain relief. In both cases, the IPG provided the stimulation ranging from 5 to 8 months before depletion. Both patients had replacement surgeries with rechargeable IPG. There was one IPG displacement in the buttock area with possible impending erosion. There was one case of SCS electrode migration with its removal and replacement with a new epidural electrode.
A total number of 16 revision surgeries were performed to manage all 14 hardware-related complications reported in 8 patients adequately. Most of these patients (5 of them) were troubled by more than one hardware-related complication episode requiring revision surgeries. Three patients had 3 revision surgeries, 2 patients had 2 revision surgeries, and 3 patients had 1 revision surgery. Among 8 patients with hardware-related complications, 3 patients had no further continuation of SCS therapy. Among these 3 patients who stopped their SCS therapy, 1 patient had 3 hardware-related episodes, and the remaining 2 patients were troubled by two hardware-related episodes before discontinuation of SCS therapy.
Due to the relatively small sample size, we did not find correlations between preoperative variables (i.e., sex, age at surgery, type of neuropathic pain, diabetes, and body mass index value) and the incidence of biological or hardware-related complications.
We treated two groups of patients affected by intractable pain due to CRPS (9 patients) or FBSS (11 patients). The mean follow-up period for all patients was 35, 4 months (range: 3–60 months). At baseline, unbearable pain intensity (VAS = 10) was reported by 9 patients (45%); 3 patients rated their pain severity as corresponding to a VAS score of 9, four reported a VAS score of 8, and 4 had a VAS score of 7. The mean pain intensity at baseline was 8.9 (range: 7–10). At short-term follow-up (3 months), the mean pain intensity was 3, 7 (range: 2–5). At the final follow-up, the mean VAS score was 4.4 (range: 2–5). The mean preoperative (ACE-III) cognitive function scores were 86.77 (range: 87–98), at 3 months 91.3 (range: 67–98), and at the last available follow-up 90 (range: 80–100).
DISCUSSION
There is no universal therapeutic procedure for patients suffering from neuropathic pain.[
In our study, 8 (40%) patients developed postoperative biological or hardware-related complications. The total incidence of SCS complications in our study is relatively high when compared to data found in the literature.[
IPG may also become displaced. Bench testing data showed that there seems to be a correlation between the site of IPG implantation and the frequency of displacements.[
A well-established solution to prevent migration is appropriate anchoring. Several titanium and plastic anchoring systems have been introduced.[
Another postoperative hardware-related complication is the incorrect functioning of the IPG. This failure was observed in 10% of cases in our study, which is a relatively high malfunction rate of implanted SCS for different pain conditions. Taylor et al.[
The incidence of electrode fracture, which did not occur in any of our patients, occurred in 3–9% of cases assessed in the literature.[
Biological complications are less frequent than hardware-related problems. Among them, the infection rate is estimated to account for 4–10% of cases.[
Postoperative complications, such as intraspinal or epidural hematomas, cerebrospinal fluid leakage, and neurological deficits, are uncommon, and they can be avoided using approaches known to improve intra-procedural safety.[
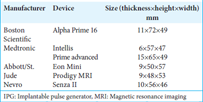
In our analysis, 5 patients (25%) developed skin erosions located above the IPG in the buttock area. These complications may have resulted due to the dimensions of the implanted high-profile IPG. There are no studies that correlate the dimensions of the implanted SCS hardware (IPG) to erosions and subsequent infection rates. High-profile IPG without the curvature of the housing may lead to skin erosions. When implanting these relatively large IPGs in the buttock area, the deeper placement of IPG may prevent its mobilization and subsequent development of skin erosions due to its displacement. The implanted Prime Advanced Sure Scan™ nonrechargeable IPG (Medtronic Inc. Minneapolis, MN, USA) had the following dimensions: 15 × 65 × 49 mm regarding thickness, height, and width. The dimensions were larger than the same nonrechargeable IPG from Boston Scientific and St Abbott implanted at that time. The relevant dimensions of Boston Scientific IPG were 11 × 72 × 49 mm and St Jude 9 × 48 × 53 mm, respectively. The dimensions of different IPG from various SCS companies are provided in
Smaller SCS devices with curved IPG shapes may greatly reduce the incidence of such complications. Verrills et al.[
The ongoing miniaturization may show the impact of larger device dimensions on the number of postoperative complications.[
Neurological injury is by far the most dreaded complication of SCS.[
Our study has several limitations. First, it was a retrospective, single-center study. Second, we used only surgically placed SCS paddle-type electrodes, which tend to be better anchored than subcutaneously placed cylindrical electrodes.[
CONCLUSION
Our results indicate that patients treated using SCS are at higher risk for the development of biological and hardware-related complications, especially skin erosions and relative infections, notably in cases when large (high-profile) IPG is utilized. The use of smaller IPG could reduce the number of these types of complications and, hence, revision surgeries.
Ethical approval
Institutional Review Board approval is not required due to the retrospective analysis of the presented clinical data.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Andersen C. Complications in spinal cord stimulation for treatment of angina pectoris. Differences in unipolar and multipolar percutaneous inserted electrodes. Acta Cardiol. 1997. 52: 325-33
2. Barolat G, Oakley JC, Law JD, North RB, Ketcik B, Sharan A. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain. Neuromodulation. 2001. 4: 59-66
3. Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg. 2014. 82: 1359-68
4. Blackburn AZ, Chang HH, DiSilvestro K, Veeramani A, McDonald C, Zhang AS. Spinal cord stimulation via percutaneous and open implantation: Systematic review and meta-analysis examining complication rates. World Neurosurg. 2021. 154: 132-43 e1
5. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J Neurosurg. 2004. 100: 254-67
6. Chaudhry ZA, Najib U, Bajwa ZH, Jacobs WC, Sheikh J, Simopoulos TT. Detailed analysis of allergic cutaneous reactions to spinal cord stimulator devices. J Pain Res. 2013. 6: 617-23
7. Choi EJ, Ri HS, Park H, Kim HJ, Yoon JU, Byeon GJ. Unexpected extrusion of the implantable pulse generator of the spinal cord stimulator-A case report. Anesth Pain Med (Seoul). 2021. 16: 103-7
8. Compton AK, Shah B, Hayek SM. Spinal cord stimulation: A review. Curr Pain Headache Rep. 2012. 16: 35-42
9. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: A review of the literature. Pain Med. 2016. 17: 325-36
10. Forouzanfar T, Kemler MA, Weber WE, Kessels AG, van Kleef M. Spinal cord stimulation in complex regional pain syndrome: Cervical and lumbar devices are comparably effective. Br J Anaesth. 2004. 92: 348-53
11. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: A prospective cohort study with long-term follow-up. Neuromodulation. 2013. 16: 523-9
12. Hord ED, Oaklander AL. Complex regional pain syndrome: A review of evidence-supported treatment options. Curr Pain Headache Rep. 2003. 7: 188-96
13. Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnée CA. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000. 343: 618-24
14. Kim DD, Vakharyia R, Kroll HR, Shuster A. Rates of lead migration and stimulation loss in spinal cord stimulation: A retrospective comparison of laminotomy versus percutaneous implantation. Pain Physician. 2011. 14: 513-24
15. Kim TH, Lee PB, Son HM, Choi JB, Moon JY. Spontaneous lead breakage in implanted spinal cord stimulation systems. Korean J Pain. 2010. 23: 78-81
16. Kinfe TM, Schu S, Quack FJ, Wille C, Vesper J. Percutaneous implanted paddle lead for spinal cord stimulation: Technical considerations and long-term follow-up. Neuromodulation. 2012. 15: 402-7
17. Krames E. Spinal cord stimulation: Indications, mechanism of action, and efficacy. Curr Rev Pain. 1999. 3: 419-26
18. Kumar K, North R, Taylor R, Sculpher M, Van den Abeele C, Gehring M. Spinal cord stimulation vs conventional medical management: A prospective randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS study). Neuromodulation. 2005. 8: 213-8
19. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: Challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006. 58: 481-96
20. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007. 132: 179-88
21. Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006. 5: 191-203
22. Levy R, Henderson J, Slavin K, Simpson BA, Barolat G, Shipley J. Incidence and avoidance of neurologic complications with paddle type spinal cord stimulation leads. Neuromodulation. 2011. 14: 412-22
23. Mammis A, Bonsignore C, Mogilner AY. Thoracic radiculopathy following spinal cord stimulator placement: Case series. Neuromodulation. 2013. 16: 443-7
24. McCabe CS, Haigh RC, Ring EF, Halligan PW, Wall PD, Blake DR. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology (Oxford). 2003. 42: 97-101
25. McGreevy K, Williams KA, Christo PJ. Cephalad lead migration following spinal cord stimulation implantation. Pain Physician. 2012. 15: E79-87
26. Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965. 150: 971-9
27. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract. 2011. 11: 148-53
28. North RB, Kidd DH, Olin JC, Sieracki JM. Spinal cord stimulation electrode design: Prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I: technical outcomes. Neurosurgery. 2002. 51: 381-9
29. Oakley JC, Krames ES, Prager JP, Stamatos J, Foster AM, Weiner R. A new spinal cord stimulation system effectively relieves chronic, intractable pain: A multicenter prospective clinical study. Neuromodulation. 2007. 10: 262-78
30. Petraglia FW, Farber SH, Gramer R, Verla T, Wang F, Thomas S. The incidence of spinal cord injury in implantation of percutaneous and paddle electrodes for spinal cord stimulation. Neuromodulation. 2016. 19: 85-90
31. Poree L, Krames E, Pope J, Deer TR, Levy R, Schultz L. Spinal cord stimulation as treatment for complex regional pain syndrome should be considered earlier than last resort therapy. Neuromodulation. 2013. 16: 125-41
32. Rabi J, Anitescu M. Late extrusion of an implantable pulse generator of a spinal cord stimulator. Pain Physician. 2016. 19: E671-4
33. Salmon J, Bates D, Du Toit N, Verrills P, Yu J, Taverner MG. Early experience with a novel miniaturized spinal cord stimulation system for the management of chronic intractable pain of the back and legs. Neuromodulation. 2023. 26: 172-81
34. Salmon J, Bates D, Du Toit N, Verrills P, Yu J, Taverner MG. Treating chronic, intractable pain with a miniaturized spinal cord stimulation system: 1-year outcomes from the AUS-nPower study during the COVID-19 pandemic. J Pain Res. 2024. 17: 293-304
35. Sears NC, Machado AG, Nagel SJ, Deogaonkar M, Stanton-Hicks M, Rezai AR. Spinal cord stimulation vs conventional medical management: A prospective randomized, controlled, multicenter study of patients with failed back surgery syndrome. Neuromodulation. 2011. 14: 312-8 discussion 318
36. Shamji MF, Paul D, Mednikov A. Minimally invasive placement of spinal cord stimulator paddle electrodes is associated with improved perioperative and long-term experience among neuropathic pain patients. Spine (Phila Pa 1976). 2018. 43: 324-30
37. Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: A systematic review and analysis of prognostic factors. Spine (Phila Pa 1976). 2005. 30: 152-60
38. Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010. 26: 463-9
39. Turner JA, Loeser JD, Bell KG. Spinal cord stimulation for chronic low back pain: A systematic literature synthesis. Neurosurgery. 1995. 37: 1088-95
40. Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996. 348: 1698-701
41. Verrills P, Vivian D, Mitchell B, Barnard A. Peripheral nerve field stimulation for chronic pain: 100 cases and review of the literature. Pain Med. 2011. 12: 1395-405
42. Villavicencio AT, Leveque JC, Rubin L, Bulsara K, Gorecki JP. Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 2000. 46: 399-405
43. Woodington BJ, Curto VF, Yu YL, Martínez-Domínguez H, Coles L, Malliaras GG. Electronics with shape actuation for minimally invasive spinal cord stimulation. Sci Adv. 2021. 7: eabg7833
44. Woźniak-Dąbrowska K, Nowacka A, Smuczyñski W, Śniegocki M. Skin allergic reaction to a spinal cord stimulation (SCS): An analysis of the world literature and a case report. Postepy Dermatol Alergol. 2020. 37: 114-6