- Department of Vascular Neurosurgery, National Institute of Neurology and Neurosurgery Manuel Velasco Suárez, Mexico City, Tlalpan, Mexico,
- National University Jorge Basadre Grohmann, Professional School of Human Medicine, Tacna, Peru.
Correspondence Address:
Edgar Nathal, Department of Vascular Neurosurgery, National Institute of Neurology and Neurosurgery, Ciudad de México, Tlalpan, Mexico.
DOI:10.25259/SNI_75_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Alejandro Serrano-Rubio1, Rodrigo López-Rodríguez1, Ambar Elizabeth Riley-Moguel1, Camilo Armando Benavides-Burbano1, Janeth N. Nuñez-Lupaca2, Alejandro Becerril-Mejía1, Rodolfo Villalobos-Diaz1, Edgar Nathal1. Bone anatomic variations of the parasellar region and its technical implications in para clinoid and posterior communicating segment aneurysms microsurgical clipping – Technical note. 08-Mar-2024;15:81
How to cite this URL: Alejandro Serrano-Rubio1, Rodrigo López-Rodríguez1, Ambar Elizabeth Riley-Moguel1, Camilo Armando Benavides-Burbano1, Janeth N. Nuñez-Lupaca2, Alejandro Becerril-Mejía1, Rodolfo Villalobos-Diaz1, Edgar Nathal1. Bone anatomic variations of the parasellar region and its technical implications in para clinoid and posterior communicating segment aneurysms microsurgical clipping – Technical note. 08-Mar-2024;15:81. Available from: https://surgicalneurologyint.com/surgicalint-articles/12786/
Abstract
Background: Microsurgical treatment of paraclinoid aneurysms is a complex task that generally requires anterior clinoid process (ACP) removal to obtain adequate surgical exposure. This procedure poses a considerable technical difficulty due to the association of the ACP to critical neurovascular structures. Furthermore, anatomical variations in the parasellar region, such as the caroticoclinoid foramen (CCF) or an interclinoid bridge (ICB), may impose additional challenges and increase surgical complications. The present study aims to briefly review some anatomic variations in the parasellar region and describe a step-by-step surgical technique for a hybrid anterior clinoidectomy based on the senior author’s experience.
Methods: We present two cases with bone variations on the parasellar region in patients with a paraclinoid aneurysm and another with a posterior communicating segment aneurysm treated by microsurgical clipping at our hospital.
Results: We focused on safely dealing with these variations during surgery, without further complications, and with good postoperative results. Patients were discharged with no significant deficit. Postoperative control, computed tomography angiography showed complete exclusion of aneurysms.
Conclusion: Although anatomical variations in the parasellar region can complicate surgical clipping of these aneurysms, it is essential to ensure the best possible surgical outcome to conduct thorough preoperative and radiological evaluations.
Keywords: Anterior clinoidectomy, Caroticoclinoid bridge, Caroticoclinoid foramen, Interclinoid bridge, Paraclinoid aneurysm
INTRODUCTION
The parasellar region is defined by all the structures that border the sella turcica and represents a crucial crossroad of essential neural and vascular structures.[
CASE DESCRIPTION
Case 1
A 71-year-old female with diabetes mellitus and systemic arterial hypertension arrived at the emergency room with exacerbated bilateral frontal thunderclap headache, associated nausea, emesis, and increased blood pressure (220/120 mmHg). On physical examination, she was awake and oriented, without motor or sensory deficit, with no abnormal findings (Glasgow Coma Scale 15 points). A head computed tomography (CT) scan showed subarachnoid hemorrhage Fisher II. CT angiography (CTA) showed three aneurysms, one of them in the right ICA posterior communicating segment, another bleb aneurysm in the choroidal segment, and another saccular aneurysm in the right M1-M2 bifurcation. Surgical clipping was performed.
Positioning
The patient is lying supine with the head angled 20° downward the vertex and rotated approximately 30° to the contralateral side. This positioning allows for an unobstructed visual axis along the sphenoid ridge to the ACP and the parasellar region.
Incision, soft-tissue dissection, craniotomy, anterior clinoidectomy
A Yasargil’s type incision was marked; an 8 cm incision at the skin-hair transition line was made, focused at the sphenoid ridge. Next, a C-shaped incision was completed in the superficial temporalis fascia and muscle, elevated, and retracted with fishhooks to expose the orbital rim and pterion. A burr hole was created at the most inferior aspect of the surgical exposure, centered over the bony indentation representing the sphenoid ridge. An oval-shaped craniotomy of 7 cm in diameter was completed, and finally, a bone chisel was used to complete the cut over the sphenoid ridge to achieve better esthetic outcomes. The sphenoid ridge and the lateral bony edge of the superior orbital fissure were removed to expose the meningo-orbital band. Then, the frontal dura is freed, and the middle fossa peeling is made using Hakuba’s technique. After that, the temporal dura propria is retracted, and the lateral dura wall of the cavernous sinus and ACP is progressively exposed. To minimize optic nerve damage by heat, a 1-mm diamond drill and Friedman and Kerrison rongeurs were used to detach the ACP from its superomedial and inferomedial attachments, exposing the optic sheath and the ICA, all this extradurally; here, we encountered a CCB, so we proceeded to remove this structure with the drill and rongeurs and constant irrigation to avoid damaging surrounding structures. Using a 1-mm diamond drill, we completed the ACP removal intradurally. Finally, distal and proximal dural rings were dissected, and the clinoid triangle was opened to completely free ICA. Adequate proximal control was obtained if necessary, and this allowed visualization of the origin and course of the posterior communicating artery. We encountered the ruptured aneurysm in the posterior communicating segment, and we employed a 15 mm permanent aneurysm clip in addition to coagulate a bleb aneurysm of the choroidal segment. Then, microdissection continued until we found the M1-M2 bifurcation aneurysm, for which we used a 6 mm curved miniclip [
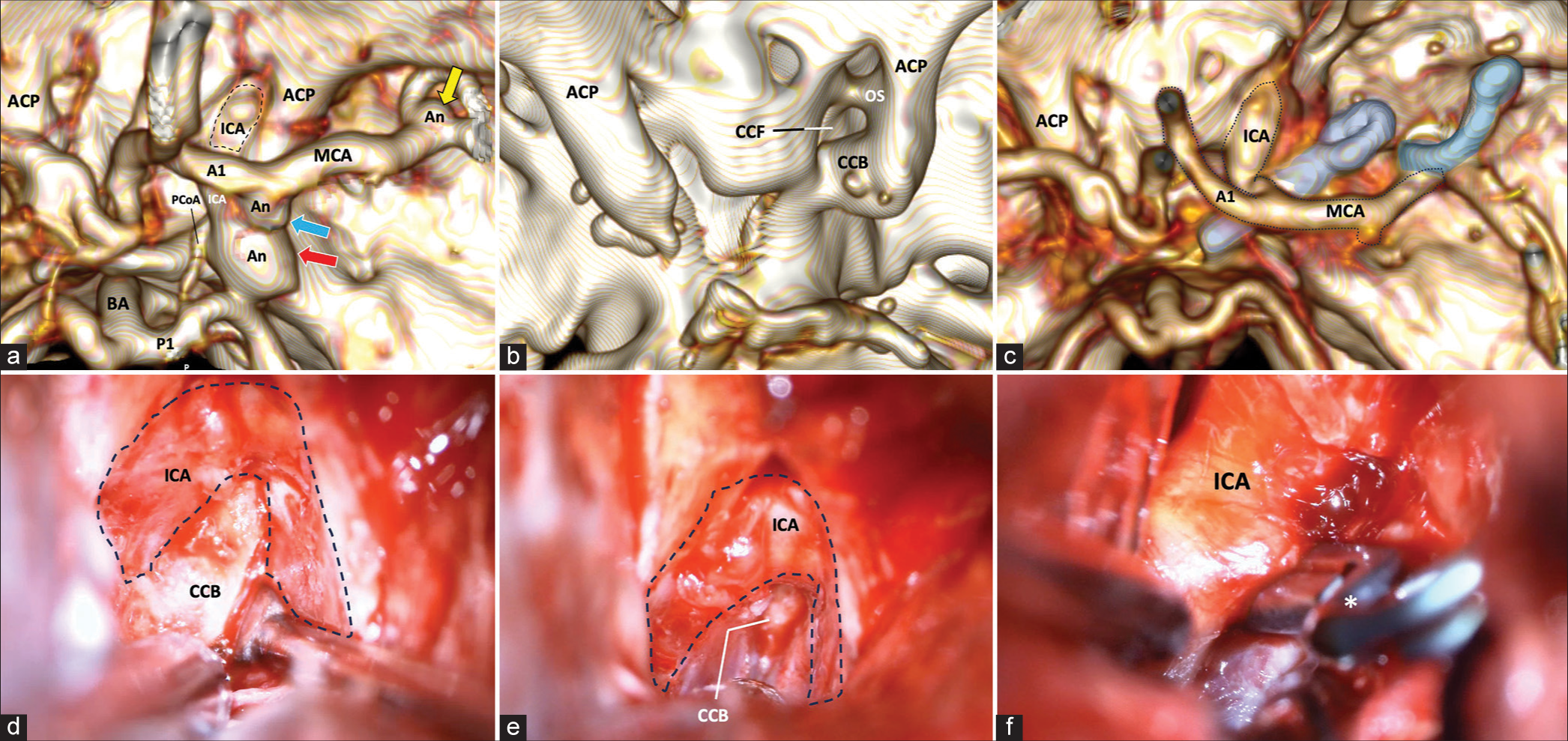
Figure 1:
(a) 3-D reconstruction of cerebral CT angiography. The location of three different aneurysms is shown. First, a saccular aneurysm (red arrow) of the ICA in the posterior communicating segment is shown, another bleb aneurysm in the ICA choroidal segment (blue arrow), and another aneurysm in the M1-M2 bifurcation (yellow arrow). (b) 3-D reconstruction of a simple CT scan of the skull. Lateral to the sellar region, we observe the presence of a CCF product of the complete closure of the CCB that circumscribes the trajectory of the ICA in its clinoid segment. (c) Postoperative 3-D reconstruction of simple CT scan. Right anterior clinoidectomy with resection of the CCB. A 15 mm straight clip occluding the aneurysm of the posterior communicating segment is observed, in addition to a 6 mm curved clip occluding the aneurysm of the M1 bifurcation. (d) Extradural approach to the sphenoidal ridge. An extradural view shows a surgical image after reaming the sphenoidal ridge with the removal of the ACP, exposing the CCB medially and circumscribing the course of the ICA in its clinoid segment. (e) In an extradural view, the fixation of the CCB on the sella turcica is observed. (f) In an intradural view, the course of the ICA is seen in its ophthalmic and posterior communicating segments. A 15 mm straight clip (asterisk) is occluding the neck of the aneurysm. CT: Computed tomography, A1: A1 segment of the anterior cerebral artery, ACP: Anterior clinoid process, An: Aneurysm, CCB: Caroticoclinoid bridge, CCF: Caroticoclinoid foramen, ICA: Internal carotid artery, ICB: Interclinoid bridge, M1: M1 segment of the middle cerebral artery, M2: M2 segment of the middle cerebral artery, MCA: Middle cerebral artery, ON: Optic nerve, OS: Optic strut, P1: P1 segment of the posterior cerebral artery, PCoA: Posterior communicating artery, BA: Basilar artery, PCP: Posterior clinoid process.
Closure
The dura was achieved using a 4-0 nylon suture. The skull base and clinoid defects were closed using a hemostatic sponge and fibrin glue. The bone flap was fixed using silk or a bone fixation device. The rest of the layers were closed with 2-0 absorbable sutures, and the skin was closed with subdermal nylon.
The patient was discharged seven days later without cranial nerve deficits, only braquiocrural hemiparesis 3-/5. Wound without signs of infection and no esthetic defects. Postoperative CTA showed complete aneurysm occlusion.
Case 2
A 49-year-old woman with systemic arterial hypertension arrived at the emergency room with an oppressive headache. She started with otalgia and tinnitus one month before. On physical examination, she was awake, oriented, and without motor or sensory deficit; CTA showed a right ventromedial paraclinoid aneurysm. Surgical treatment was performed.
Positioning
We used a similar position described in Case 1.
Craniotomy, anterior clinoidectomy
An asymmetrical oval-shaped craniotomy measuring 7 cm in diameter is completed. The superior orbital fissure border was removed to expose the meningo-orbital band. Then, the middle fossa peeling was performed using Hakuba’s technique to retract the lateral dura wall of the cavernous sinus and progressively expose the ACP. Friedman and Kerrison Rongeurs were used to detach the ACP from its superomedial and inferomedial attachments, exposing the optic sheath and the ICA. A linear dural incision toward the optic sheath, followed by cerebrospinal fluid drainage from the optico-carotid cistern for brain relaxation. Using a 1-mm diamond drill, we completed the ACP removal intradural. Here, we encountered an ICB and proceeded to drill this to have a full view of the aneurysm neck with ventromedial dome projection in addition to opening the distal dural ring for carotid luxation and adequate clipping of the aneurysm, preserving the patency of the carotid artery; this maneuver allowed us to put 290°-angulated fenestrated 5 mm clips. A visual control with fluorescein confirmed the patency of parent vessels and complete closure of aneurysms [
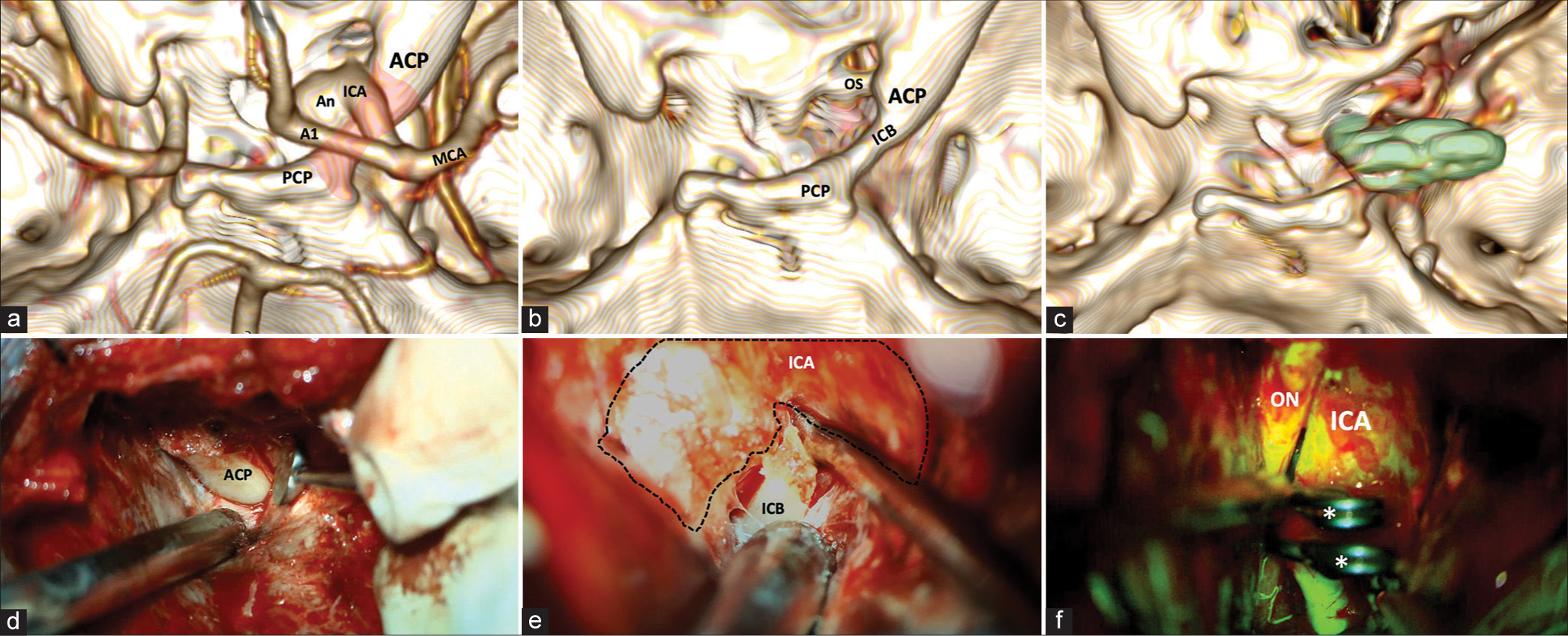
Figure 2:
(a) 3-D reconstruction of cerebral CT angiography. The vascular relationships concerning the ICB (red shading) on the right side are highlighted from a panoramic view centered on the middle fossa. (b) 3-D reconstruction of a simple CT scan of the skull. The complete course of the ICB is seen in the absence of vascular components. (c) Postoperative 3-D reconstruction of simple CT scan. Right anterior clinoidectomy with resection of the ICB. The placement of 2 right-angled 5 mm fenestrated clips with the tips pointing toward the OS is observed (green shading). (d) Extradural approach to the sphenoidal ridge. An extradural view shows a surgical image after reaming the sphenoidal ridge. (e) Intradural view. A surgical image exposes the ICB and its relation with the ICA in its ophthalmic segment. (f) Fluorescein video angiography (FL-VAG) post clipping. Two 5-mm right-angled fenestrated clips placed over the ICA in its ophthalmic segment with adequate patency are observed. CT: Computed tomography, A1: A1 segment of the anterior cerebral artery, ACP: Anterior clinoid process, An: Aneurysm, ICA: Internal carotid artery, ICB: Interclinoid bridge, M1: M1 segment of the middle cerebral artery, MCA: Middle cerebral artery, ON: Optic nerve, OS: Optic strut, PCP: Posterior clinoid process.
Closure
The closure technique is similar to that described in Case 1. The patient was discharged six days later, remaining only with incomplete III nerve palsy. No defects or complications regarding the wound. Postoperative CTA control also showed complete exclusion of aneurysms.
DISCUSSION
The presence of anatomic variations in the parasellar region, such as a CCF, ICB, and/or pneumatization of the ACP, may further increase the complexity of this procedure, and modifications of the technique are usually necessary to avoid surgical complications.[
CONCLUSION
Conducting thorough preoperative assessments and radiological evaluations is essential to ensuring the best surgical outcome in cases of microsurgical aneurysm clipping. Each of these variants may limit the complete resection of the ACP by the extradural approach. Based on CT angiographic observations, the authors recommend modifications to the standard anterior clinoidectomy procedure to enhance surgical access, reduce the risk of complications, and achieve improved patient outcomes.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Das S, Suri R, Kapur V. Ossification of caroticoclinoid ligament and its clinical importance in skull-based surgery. Sao Paulo Med J. 2007. 125: 351-3
2. Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983. 58: 824-31
3. Erturk M, Kayalioglu G, Govsa F. Anatomy of the clinoidal region with special emphasis on the caroticoclinoid foramen and interclinoid osseous bridge in a recent Turkish population. Neurosurg Rev. 2004. 27: 22-6
4. Fernandez-Miranda JC, Tormenti M, Latorre F, Gardner P, Snyderman C. Endoscopic endonasal middle clinoidectomy: Anatomic, radiological, and technical note. Neurosurgery. 2012. 71: ons233-9 discussion ons239
5. Kier EL. Embryology of the normal optic canal and its anomalies. An anatomic and roentgenographic study. Invest Radiol. 1966. 1: 346-62
6. Nikolova S, Toneva D, Zlatareva D, Fileva N. Osseous bridges of the sphenoid bone: Frequency, bilateral and sex distribution. Biology (Basel). 2023. 12: 492
7. Ota N, Tanikawa R, Miyazaki T, Miyata S, Oda J, Noda K. Surgical microanatomy of the anterior clinoid process for paraclinoid aneurysm surgery and efficient modification of extradural anterior clinoidectomy. World Neurosurg. 2015. 83: 635-43
8. Piagkou M, Fiska A, Tsakotos G, Triantafyllou G, Politis C, Koutserimpas C. A morphological study on the sphenoid bone ligaments’ ossification pattern. Surg Radiol Anat. 2023. 45: 1405-17
9. Quiñones-Hinojosa A, Schmidek HH, editors. Chapter: 46. Surgical treatment of paraclinoid aneurysms. Schmidek and sweet: Operative neurosurgical techniques. Netherlands: Elsevier; 2022. p.
10. Ruscalleda J. Imaging of parasellar lesions. Eur Radiol. 2005. 15: 549-59
11. Sharma A, Rieth GE, Tanenbaum JE, Williams JS, Ota N, Chakravarthi S. A morphometric survey of the parasellar region in more than 2700 skulls: Emphasis on the middle clinoid process variants and implications in endoscopic and microsurgical approaches. J Neurosurg. 2018. 129: 60-70
12. Singh R. Carotico-clinoid foramen and associated clinical significance: Comprehensive review. Cureus. 2021. 13: e12828
13. Skandalakis GP, Koutsarnakis C, Pantazis N, Kalyvas A, Komaitis S, Lani E. Caroticoclinoid bar: A systematic review and meta-analysis of its prevalence and potential implications in cerebrovascular and skull base surgery. World Neurosurg. 2019. 124: 267-76
14. Suprasanna K, Kumar A. Surgically relevant bony anatomical variations in paraclinoid aneurysms-three-dimensional multi-detector row computed tomography-based study. J Neurosci Rural Pract. 2017. 8: 330-4
15. Zdilla MJ, Cyrus LM, Lambert HW. Surgical neurology international carotico-clinoid foramina and a double optic canal: A case report with neurosurgical implications. Surg Neurol Int. 2015. 6: 13
16. Zhao X, Labib MA, Avci E, Preul MC, Baskaya MK, Little AS. Navigating a carotico-clinoid foramen and an interclinoidal bridge in the endonasal endoscopic approach: An anatomical and technical note. J Neurol Surg B Skull Base. 2021. 82: 534-9