- Department of Neurosurgery, Kagawa Rosai Hospital, Marugame, Kagawa, Japan
Correspondence Address:
Atsuhito Uneda
Department of Neurosurgery, Kagawa Rosai Hospital, Marugame, Kagawa, Japan
DOI:10.4103/2152-7806.186509
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Uneda A, Suzuki K, Okubo S, Hirashita K, Yunoki M, Yoshino K. Brain abscess caused by Nocardia asiatica. Surg Neurol Int 18-Jul-2016;7:74
How to cite this URL: Uneda A, Suzuki K, Okubo S, Hirashita K, Yunoki M, Yoshino K. Brain abscess caused by Nocardia asiatica. Surg Neurol Int 18-Jul-2016;7:74. Available from: http://surgicalneurologyint.com/surgicalint_articles/brain-abscess-caused-nocardia-asiatica/
Abstract
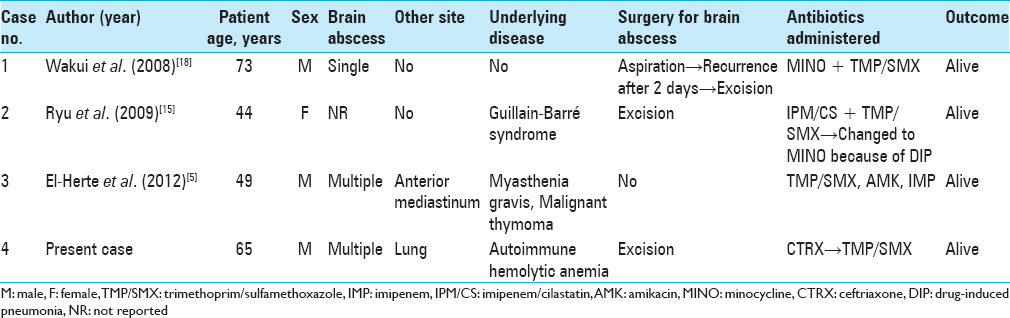
Background:Nocardia infection of the central nervous system leading to brain abscess is a rare condition but has a high mortality rate. Among the species of Nocardia, only three cases of brain abscess due to Nocardia asiatica infection have been reported.
Case Description:A 65-year-old man with a history of autoimmune hemolytic anemia treated with prednisolone presented to our hospital because of occipital headache. Brain magnetic resonance imaging showed bilateral occipital lesions. The patient underwent craniotomy and resection of the left occipital lobe lesion. N. asiatica was identified by 16S rRNA sequencing of the resected specimen. Treatment with trimethoprim/sulfamethoxazole led to a complete resolution of the brain lesion.
Conclusion:Because of the different antimicrobial sensitivity patterns among Nocardia species, both appropriate subtyping and susceptibility testing of uncommon species such as N. asiatica are required for the successful treatment of nocardial infections.
Keywords: Brain abscess, Nocardia asiatica, 16S rRNA
INTRODUCTION
Nocardia infection of the central nervous system (CNS) leading to brain abscess is rare but has a high mortality rate.[
CASE REPORT
A 65-year-old man with a history of autoimmune hemolytic anemia treated with prednisolone (10 mg/day for years) presented to our hospital because of occipital headache of 1-week duration. There was no fever and his neurological examination findings were normal. The results of the laboratory tests performed on admission were normal. Brain magnetic resonance imaging (MRI) showed bilateral occipital lesions. The central part of the mass was hypointense on T1-weighted imaging (T1WI) and hyperintense on T2-weighted imaging (T2WI) [Figure
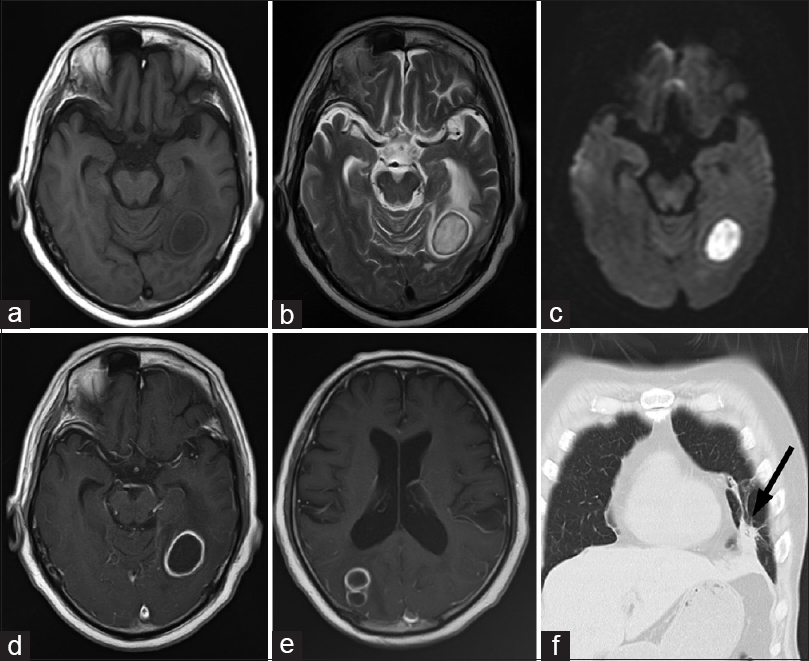
Figure 1
Preoperative images. (a) Hypointensity of the lesion and the isointensity of its peripheral rim are seen on axial T1-weighted imaging (T1WI). (b) Hyperintensity of the lesion and the hypointensity of its peripheral rim are seen on axial T2-weighted imaging (T2WI). (c) Hyperintense signals obtained using diffusion-weighted imaging. (d and e) Bilateral ring-enhanced lesions are seen on axial T1WI following the administration of Gd-DTPA as contrast agent. (f) A small nodule in the lingular segment of the left lung (arrow) is revealed by coronal thoracic computed tomography
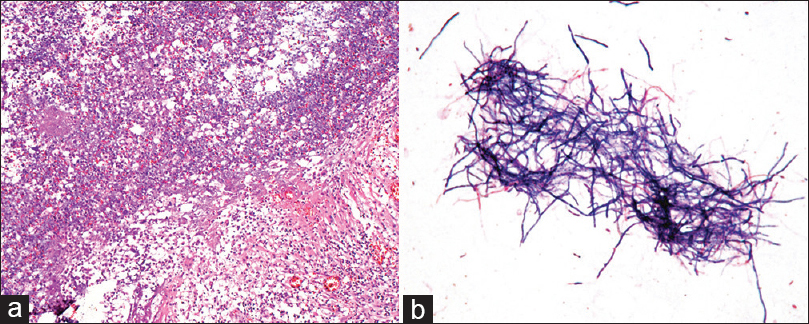
Figure 2
(a) Photomicrograph of the hematoxylin and eosin stained resected specimen shows numerous inflammatory cells around the abscess cavity, with a surrounding rim of fibrosis. (×100). (b) Smears from the capsule culture yielded gram-positive branching filaments, raising suspicion of a Nocardia species as the infectious agent
DISCUSSION
Nocardia species are gram-positive, partially acid-fast, and strictly aerobic branching filamentous bacteria.[
CNS nocardiosis most frequently manifests as brain abscesses, however, in rare cases as meningitis or spinal cord infections.[
The standard medical treatment for Nocardia brain abscesses is trimethoprim/sulfamethoxazole.[
Diagnosis is mainly based on bacteriological cultures; 82% of patients are diagnosed with cultures of aspirates from the site of infection, 31% based on the biopsy specimen, and 8.3% following cerebrospinal fluid (CSF) culture.[
Sequencing of the 16S rRNA gene of Nocardia has become a valuable tool for accurate species-level identification. In fact, application of this method has resulted in the identification of new species of Nocardia.[
In summary, the appropriate subtyping and susceptibility determination of uncommon species of Nocardia, such as N. asiatica, are necessary for the treatment of nocardial infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anagnostou T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis E. Nocardiosis of the central nervous system: Experience from a general hospital and review of 84 cases from the literature. Medicine. 2014. 93: 19-32
2. Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Infect Immun. 1994. 62: 1787-98
3. Bharadwaj R, Swaminathan S, Salimnia H, Fairfax M, Frey A, Chandrasekar PH. Clinical impact of the use of 16S rRNA sequencing method for the identification of “difficult-to-identify” bacteria in immunocompromised hosts. Transpl Infect Dis. 2012. 14: 206-12
4. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006. 19: 259-82
5. El-Herte RI, Kanj SS, Araj GF, Chami H, Gharzuddine W. First Report of Nocardia asiatica Presenting as an Anterior Mediastinal Mass in a Patient with Myasthenia Gravis: A Case Report and Review of the Literature. Case Rep Infect Dis 2012. 2012. p.
6. Iona E, Giannoni F, Brunori L, de Gennaro M, Mattei R, Fattorini L. Isolation of Nocardia asiatica from cutaneous ulcers of a human immunodeficiency virus-infected patient in Italy. J Clin Microbiol. 2007. 45: 2088-9
7. Kageyama A, Poonwan N, Yazawa K, Mikami Y, Nishimura K. Nocardia asiatica sp. nov., isolated from patients with nocardiosis in Japan and clinical specimens from Thailand. Int J Syst Evol Microbiol. 2004. 54: 125-30
8. Leitner E, Valentin T, Hoenigl M, Lanz P, Flick H, Zollner-Schwetz I. First report of Nocardia asiatica olecranon bursitis in an immunocompetent traveler returning to Austria. J Clin Microbiol. 2013. 51: 2461-2
9. Lerner PI. Nocardiosis. Clin Infect Dis. 1996. 22: 891-903
10. Lin YJ, Yang KY, Ho JT, Lee TC, Wang HC, Su FW. Nocardial brain abscess. J Clin Neurosci. 2010. 17: 250-3
11. Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: Treatment strategies and factors influencing outcome. Neurosurgery. 1994. 35: 622-31
12. Matsumoto T, Shimizu T, Aoshima Y, Oda H, Kikuchi K, Katsura H. Endobronchial hamartoma with obstructive pneumonia due to Nocardia asiatica. Gen Thorac Cardiovasc Surg. 2011. 59: 141-4
13. McNeil MM, Brown JM. The medically important aerobic actinomycetes: Epidemiology and microbiology. Clin Microbiol Rev. 1994. 7: 357-417
14. Patil A, Cherian A, Iype T, Sandeep P. Nocardial brain abscess in an immunocompetent individual. Neurol India. 2011. 59: 779-82
15. Ryu A, Kawahara R, Mori K, Tamura M, Nakano K, Yoshioka A. A Case of Brain Abscess caused by Nocardia asiatica. J Jpn Soc Clin Microbiol. 2009. 19: 163-70
16. Saraya T, Ohkuma K, Kikuchi K, Tamura M, Honda K, Yamada A. Cytomegalovirus pneumonia in a patient with interstitial pneumonia and Nocardia asiatica presenting as cavitary lung lesions. Intern Med. 2013. 52: 593-7
17. Verfaillie L, De Regt J, De Bel A, Vincken W. Nocardia asiatica visiting Belgium: Nocardiosis in a immunocompetent patient. Acta Clin Belg. 2010. 65: 425-7
18. Wakui D, Ito H, Ikeda R, Yoshida Y, Furuya Y, Tanaka K. A complicated case of Nocardia brain abscess for differential diagnosis. No Shinkei Geka. 2008. 36: 1011-6
19. Wallace RJ, Tsukamura M, Brown BA, Brown J, Steingrube VA, Zhang YS. Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J Clin Microbiol. 1990. 28: 2726-32