- Department of Neurosurgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland,
- Department of Neurosurgery, Maastricht University Medical Center, Maastricht, Netherlands,
- Department of Pathology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Correspondence Address:
Anselmi Kovalainen, Department of Neurosurgery, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
DOI:10.25259/SNI_621_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anselmi Kovalainen1, Roel Haeren1,2, Anders Paetau3, Martin Lehecka1. Brainstem intraparenchymal schwannoma: A case report and literature review. 11-Oct-2021;12:508
How to cite this URL: Anselmi Kovalainen1, Roel Haeren1,2, Anders Paetau3, Martin Lehecka1. Brainstem intraparenchymal schwannoma: A case report and literature review. 11-Oct-2021;12:508. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11175
Abstract
Background: Intracranial intraparenchymal schwannomas (IS) are rare tumors that have mainly been described in case reports. Here, we report on a case of a brainstem IS and included a comprehensive literature review.
Case Description: A 74-year-old man presented with progressive gait disturbances. CT- and MRI-imaging revealed a contrast-enhancing mass accompanied by a cyst in the dorsolateral pons. Hemangioblastoma was suspected and surgery was advised. During surgery, gross total resection of a non-invasive tumor was performed. Postoperative recovery was uneventful. Based on histopathological examination, the intraparenchymal brainstem tumor was diagnosed as schwannoma.
Conclusion: Our extensive review illustrates that ISs are benign tumors that most often present in relatively young patients. Malignant cases have been described but form an extremely rare entity. Preoperative diagnosis based on radiological features is difficult but should be considered when peritumoral edema, calcifications, and cysts are noted. In benign cases, gross total resection of the lesion is curative. To adequately select this treatment and adjust the surgical strategy accordingly, it is important to include IS in the preoperative differential diagnosis when the abovementioned radiological features are present.
Keywords: Brainstem, Case report, Intraparenchymal, Review, Schwannoma, Tumor
INTRODUCTION
Schwannomas are tumors that originate from Schwann cells, which form the myelin sheath of peripheral nerves.[
CASE REPORT
A 74-year-old man with no reported prior medical condition presented with progressive gait disturbances and hearing loss that had developed over a few months. Neurological examination revealed sensory asymmetry in the left upper and middle trigeminal branch areas, broad-based gait, diplopia, dysphagia, and dysarthric speech. Imaging studies showed a cystic tumor in the left dorsolateral pons [
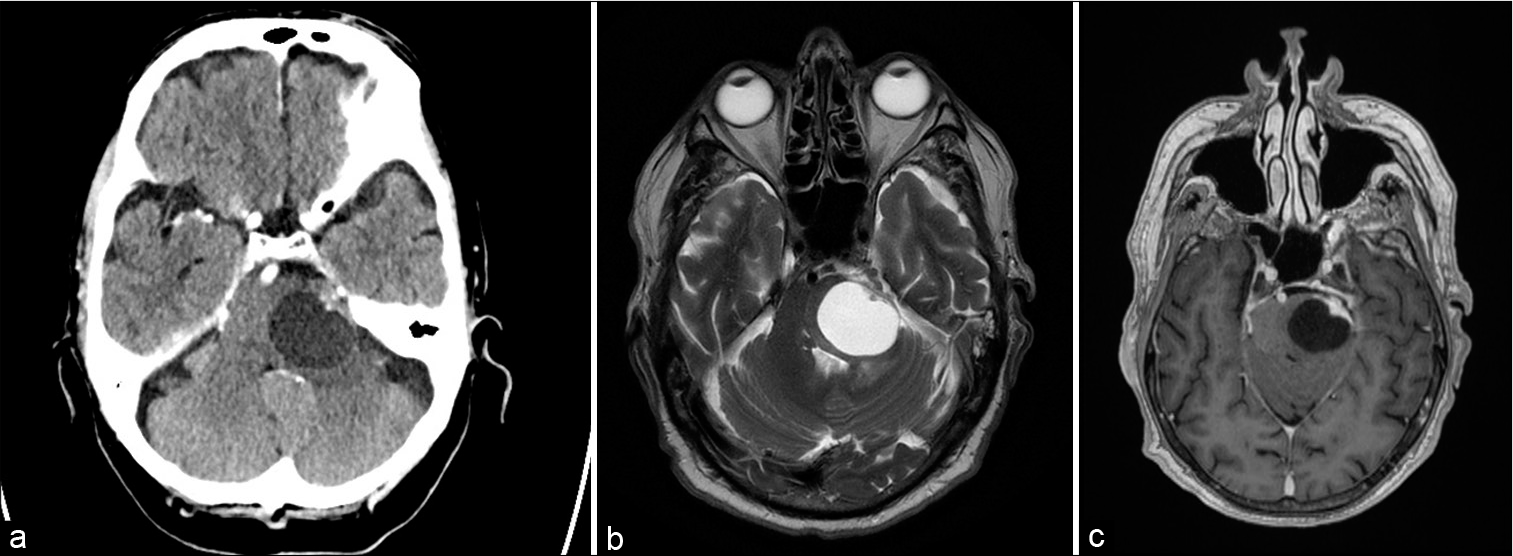
Figure 1:
Preoperative radiological images. CT-scan of the brain without contrast showed a sharply delineated cystic lesion located in the left dorsolateral pons (a). There appear some calcifications located in the anterolateral solid mass of the lesions. Contrast-enhanced T2-weighed MRI images again showing the hyperintense aspect of the cystic fluid (b). Limited edema can be noted at the dorsal side of the tumor. On the contrast-enhanced T1-weighted images, the tumor mass and part of the cyst wall enhanced homogenously (c).
A left-sided suboccipital retrosigmoid craniotomy was performed [
Video 1
Histological assessment of the tumor sections showed clusters of spindle cells surrounded by fascicles and palisades in addition to thick-walled vessels [
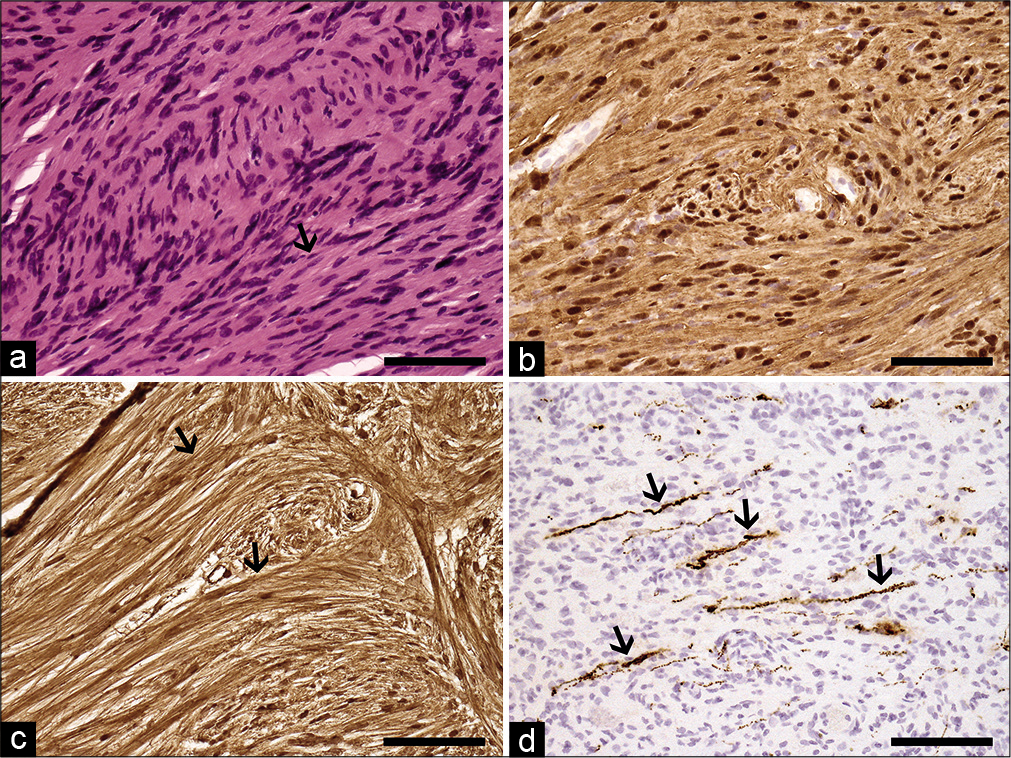
Figure 2:
Histopathological images. Following HE-staining, elongated spindle cells (arrow) are noted in the biopsy sections (a). Additional immunohistochemical staining revealed a strong positivity for S-100 protein (b) and pericellular basement membranes (arrows) of tumor cells following collagen IV staining (c). In figure 2D, a scattered neurofilament-positive intratumoral axons (arrows) can be seen. Based on these findings, the tumor was diagnosed as of intraparenchymal schwannoma Grade I. Original magnification ×200 in all pictures, 100 mm scale bar.
DISCUSSION
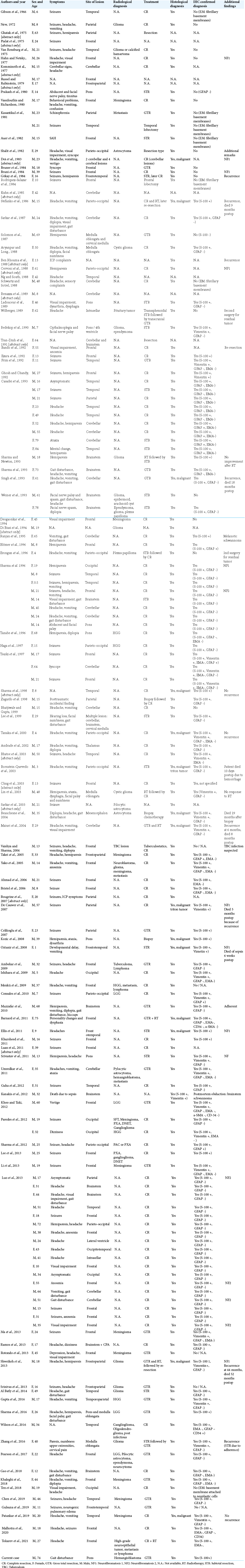
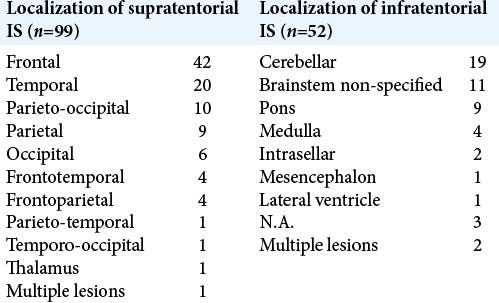
IS s are rare intracranial intra-axial tumors. We have found 150 cases reporting on histopathological confirmed IS [
Clinical characteristics
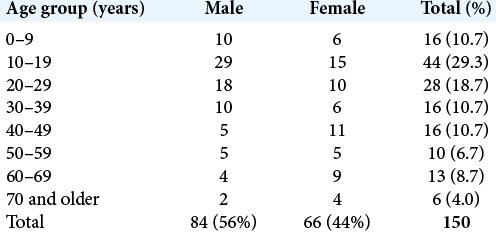
ISs present at a relatively young age, with a majority occurring before the age of 30 and a slight male predominance [
Clinical presentation and clinical course are mostly dependent on tumor location and increased intracranial pressure. Tumors involving functional areas may be associated with a relatively short time between presentation and diagnosis.[
Histogenesis
Schwannomas originate from Schwann cells, which form the myelin sheath of peripheral nerves.[
In our case, one could suggest a relation of the tumor with Schwann cells of the trigeminal nerve. However, the radiological findings suggested an intraparenchymal origin of the tumor as there was no border between the brainstem and the tumor. In addition, the tumor was located within the brainstem parenchyma as observed intraoperatively. If the tumor was related to trigeminal nerve Schwann cells, one would have expected a capsule between the Schwannoma and the brainstem which was not apparent in this case. Since the intraparenchymal myelin covering of the trigeminal nerve is dependent on astrocytes, and not Schwann cells, it is unlikely that the tumor is directly related to the trigeminal nerve.[
Radiological features
Diagnosis of IS based on preoperative radiological examinations is difficult. Our review revealed that a wide variety of differential diagnoses were suspected preoperatively and IS was not considered in any of these cases [
In contrast, cranial nerve Schwannomas are radiologically characterized by a heterogeneous hyperintensity in T2-weighted images, with deformation of adjacent parenchyma, neural cisterns and bony foramina, and have a clear relation to a cranial nerve. Moreover, cranial nerve Schwannomas usually have a well delineated margin from the brainstem parenchyma and cause minimal peritumoral edema.[
Histopathological findings
Histological evaluation of IS shows a typical biphasic tissue pattern of Antoni type A and B areas.[
Treatment and prognosis
Since IS are mostly benign lesions, gross total or complete resection of the tumor is usually curative. Therefore, surgical resection is the preferred treatment for symptomatic lesions.[
Although rare, IS should be included in the differential diagnosis when typical radiological features are present. This is relevant as surgical approach and technique may be different in comparison to the many differential diagnoses that are included in [
The role of radiotherapy or chemotherapy as primary treatment of benign IS remains unknown. We found two cases in which radiotherapy was given as primary treatment.[
CONCLUSION
ISs are rare benign tumors occurring mostly in young patients. Clinical presentation is usually related to tumor location or increased intracranial pressure. Gross total resection of the lesion is curative. To adjust the surgical strategy accordingly, ISs should be considered preoperatively when radiological features such as peritumoral edema, calcifications, and cysts are noted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on:
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on:
www.surgicalneurologyint.com
Acknowledgments
None.
References
1. Casadei GP, Komori T, Scheithauer BW, Miller GM, Parisi JE, Kelly PJ. Intracranial parenchymal schwannoma. J Neurosurg. 2009. 79: 217-22
2. Chen F, Zhao S, Yu Y, Chen D. Intraparenchymal schwannoma with calcification of the temporal lobe: Case report and literature review. Medicine (Baltimore). 2019. 98: e14263
3. de Cauwer H, Bogers JP, Duwel V, van den Hauwe, Croese P, van Marck E. An intracerebral intraparenchymatous triton tumor in a man with neurofibromatosis. J Neurol. 2007. 254: 1009-11
4. Erongun U, Özkal E, Acar O, Uygun A, Kocaoğullar Y, Güngör S. Intracerebral schwannoma: Case report and review. Neurosurg Rev. 1996. 19: 269-74
5. Gibson AA, Hendrick EB, Conen PE. Intracerebral schwannoma. Report of a case. J Neurosurg. 1966. 24: 552-7
6. Gökay H, Izgi N, Barlas O, Erseven G. Supratentorial intracerebral schwannomas. Surg Neurol. 1984. 22: 69-72
7. Khoo HM, Taki T. Periventricular intraparenchymal schwannoma. Case report. Neurol Med Chir (Tokyo). 2012. 52: 603-7
8. Khursheed N, Rumana M, Ramzan A, Furqan N, Abrar W, Salma B. Frontal intraparenchymal schwannoma. J Clin Neurosci. 2011. 18: 411-3
9. Kozić D, Nagulic M, Samardžić M, Ostojic J, Rasulić L, Cvetković-Dožić D. Intrapontine malignant nerve sheath tumor: MRI and MRS features. Acta Neurol Belg. 2008. 108: 67-71
10. Lin J, Feng H, Li F, Zhao B, Guo Q. Intraparenchymal schwannoma of the medulla oblongata: Case report. J Neurosurg. 2003. 98: 621-4
11. Luo W, Ren X, Chen S, Liu H, Sui D, Lin S. Intracranial intraparenchymal and intraventricular schwannomas: Report of 18 cases. Clin Neurol Neurosurg. 2013. 115: 1052-7
12. Mahore A, Kansal R, Epari S, Kataria N, Sharma P. Supratentorial intraparenchymal schwannoma mimicking a glial tumor. Neurol India. 2012. 60: 335-7
13. Menkü A, Öktem IS, Kontaş O, Akdemir H. Atypical intracerebral schwannoma mimicking glial tumor: Case report. Turk Neurosurg. 2009. 19: 82-5
14. Muzzafar S, Ketonen L, Weinberg JS, Schellingerhout D. Imaging and clinical features of an intra-axial brain stem schwannoma. Am J Neuroradiol. 2010. 31: 567-9
15. Paredes I, Jimenez Roldán L, Ramos A, Lobato RD, Ricoy JR. Intraparenchymal schwannomas: Report of two new cases studied with MRI and review of the literature. Clin Neurol Neurosurg. 2012. 114: 42-6
16. Peker S, Kurtkaya O, Uzün I, Pamir MN. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery. 2006. 59: 354-9
17. Sharma V, Newton G. Schwannoma of the medulla oblongata. Br J Neurosurg. 1993. 7: 427-9
18. Shweikeh F, Drazin D, Bannykh SI. Malignant intracerebral nerve sheath tumors: A case report with review of the literature. Case Rep Surg. 2013. 2013: 384076
19. Skolnik AD, Loevner LA, Sampathu DM, Newman JG, Lee JY, Bagley LJ. Cranial nerve schwannomas: Diagnostic imaging approach. Radiographics. 2016. 36: 1463-77
20. Tsuiki H, Kuratsu J, Ishimaru Y, Nakahara T, Kishida K, Takamura M. Intracranial intraparenchymal schwannoma: Report of three cases. Acta Neurochir (Wien). 1997. 139: 756-60
21. Wilberger JE. Primary intrasellar schwannoma: Case report. Surg Neurol. 1989. 32: 156-8
22. Zhang Q, Ni M, Liu WM, Jia W, Jia GJ, Zhang JT. Intra-and extramedullary dumbbell-shaped schwannoma of the medulla oblongata: A case report and review of the literature. World Neurosurg. 2017. 98: 873.e1-7