- Department of Interventional Neuroradiology, Royal Preston Hospital, Lancashire, United Kingdom.
Correspondence Address:
Rukhtam Saqib, Department of Interventional Neuroradiology, Royal Preston Hospital, Lancashire, United Kingdom.
DOI:10.25259/SNI_991_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rukhtam Saqib, Siddhartha Wuppalapati, Hemant Sonwalkar, Karthikeyan Vanchilingam, Somenath Chatterjee, Gareth Roberts, Nihal Gurusinghe. Can further subdivision of the Raymond-Roy classification of intracranial aneurysms be useful in predicting recurrence and need for future retreatment following endovascular coiling?. 29-Apr-2022;13:170
How to cite this URL: Rukhtam Saqib, Siddhartha Wuppalapati, Hemant Sonwalkar, Karthikeyan Vanchilingam, Somenath Chatterjee, Gareth Roberts, Nihal Gurusinghe. Can further subdivision of the Raymond-Roy classification of intracranial aneurysms be useful in predicting recurrence and need for future retreatment following endovascular coiling?. 29-Apr-2022;13:170. Available from: https://surgicalneurologyint.com/surgicalint-articles/11574/
Abstract
Background: The Raymond-Roy classification has been the standard for neck recurrences following endovascular coiling with three grades. Several modified classification systems with subdivisions have been reported in literature but it is unclear whether this adds value in predicting recurrence or retreatment. Our aim is to assess if these subdivisions aid in predicting recurrence and need for retreatment.
Methods: A retrospective review of all patients undergoing endovascular coiling between 2013 and 2014. Patients requiring stent assistance or other embolization devices were excluded from the study. The neck residue was graded at time of coiling on the cerebral angiogram and subsequent 6, 24, and 60 months MRA. Correlation between grade at coiling and follow-up with need for subsequent retreatment was assessed.
Results: Overall, 17/200 (8.5%) cases required retreatment within 5 years of initial coiling. 4/130 (3.1%) required retreatment within 5 years with initial Grade 0 at coiling, 6/24 cases (25%) of those Grade 2a, 4/20 cases (20%) Grade 2b, 3/8 (38%) Grade 3, and none of those with Grade 1. Large aneurysms ≥11 mm had an increased risk of aneurysm recurrence and retreatment. About 9.7% of ruptured aneurysms required retreatment versus 4.4% for unruptured. About 55% of carotid ophthalmic aneurysms were retreated.
Conclusion: Although the modified classification system was significantly predictive of progressive recurrence and need for retreatment, no significant difference between the subdivisions of Grade 2 was observed. Similar predictive value was seen when using the Raymond-Roy classification compared to the new modified, limiting the usefulness of the new system in clinical practice.
Keywords: Coiling, Intracranial aneurysms, Raymond-Roy, Recurrence
INTRODUCTION
Endovascular treatment (EVT) of intracranial aneurysms has become the treatment norm over surgical clipping for both ruptured and unruptured aneurysms since the International Subarachnoid Aneurysm Trial (ISAT).[
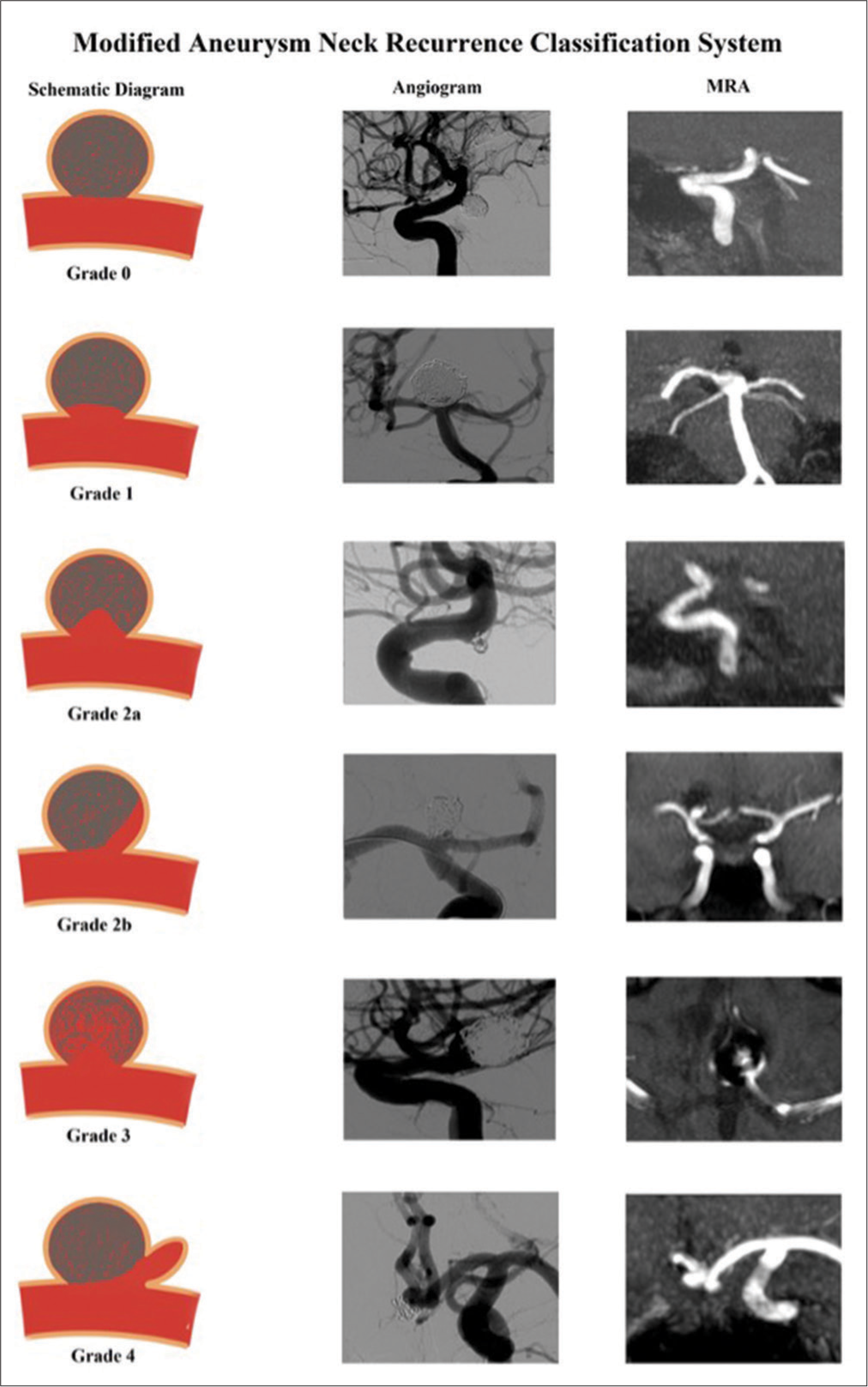
Our modified classification system is a similar version published in recent literature.[
Our aim was to assess whether the modified aneurysm neck recurrence classification system can predict progressive aneurysm recurrence and subsequent retreatment.
MATERIALS AND METHODS
Patient selection
A retrospective review of all patients whom underwent endovascular coiling at our institution between 2013 and 2014 was conducted. This period was chosen to ensure follow-up was available for patients upto 5 years post procedure at the time of the study. Both ruptured and unruptured aneurysms were included in the study. Cases which required stent assistance or involved the use of other embolization devices, such as Webs, were excluded from the study. It is our routine practice to use a balloon in the majority of endovascular coiling case. If a patient had more than 1 aneurysm treated during the study period, each aneurysm was included as a separate case. The procedures were performed by two experienced consultant interventional neuroradiologists. All procedures were performed on a Philips Allura FD20 biplane. Follow-up imaging was performed on 3T MRA time-of-flight imaging, with inclusion of additional standard diagnostic sequences.
Data collection and analysis
Using the initial cerebral digital subtraction angiogram, the location, size, side, and ruptured status of the aneurysm were noted. The aneurysm size was divided into small (≤5 mm), medium (6–10 mm), and large (≥11 mm).
The grade of the neck residue was assessed on the initial coiling angiogram DSA. In our practice, we follow up patients with a 6-month and 24-month time-of-flight 3T MRA. One of the three vascular neurosurgeons also performs a 60-month MRA follow-up; these were also reviewed. The neck recurrence was assessed using the modified and Raymond-Roy aneurysm recurrence classification system on each subsequent follow-up MRA. Each scan was reviewed an interventional neuroradiologist who had no involvement in the procedure to reduce bias followed by a reviewed by a consultant interventional neuroradiologist. Any disagreements of classification were discussed among the other authors, with the majority agreement being the final classification. For patients with MRA findings concerning for recurrence, a cerebral angiogram DSA was performed to confirm and patient retreated if required. Progression of neck grade recurrence was analyzed from the time coiling as baseline to 6, 24, and 60 months. Change in aneurysm recurrences between 6–24 months and 24–60 months was also assessed separately. A Chi-square test was performed to assess for correlation.
For cases which required retreatment of aneurysm recurrence, the method of retreatment and any subsequent recurrence of the neck residue within the 1st year post retreatment were noted. Factors such as the size of aneurysm, location, and whether the aneurysm was ruptured or unruptured were analyzed for any correlation.
RESULTS
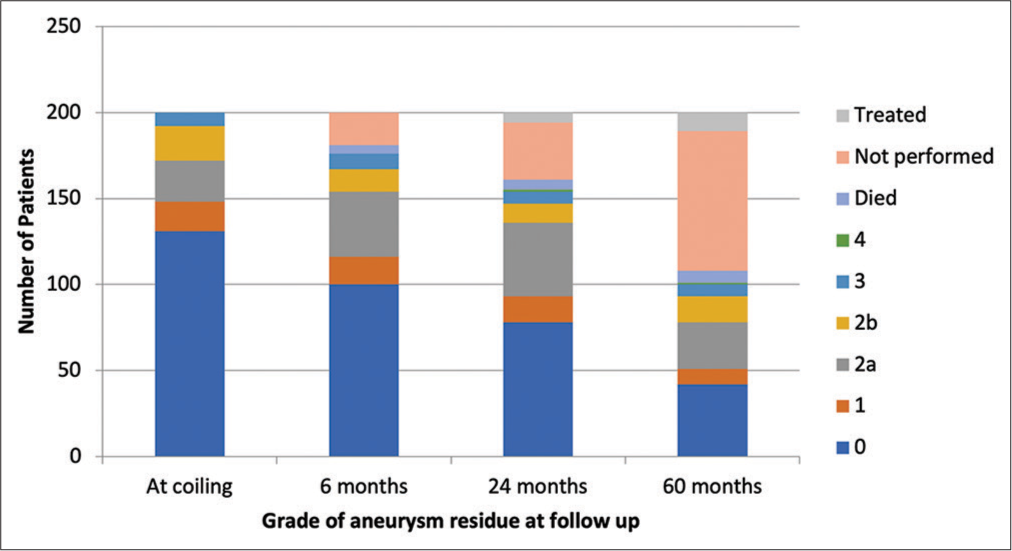
A total of 200 cases were performed within the 2-year period. One hundred aneurysms were ≤5 mm, 77 aneurysms were between 6 and 10 mm, and 23 were ≥11 mm. Fifty-six aneurysms were on the left side, 74 aneurysms on the right side, and 70 aneurysms did not have a side as they occurred in a single vessel such as basilar artery or anterior communicating artery (ACOM). One hundred and forty-four were female and 56 were male. Forty-five were unruptured and 155 ruptured aneurysms. Ninety of the procedures were performed by one consultant and the remaining 110 by the other. The aneurysm locations were 12 anterior cerebral artery, 55 ACOM, four anterior choroidal artery, 13 basilar top, nine carotid ophthalmic, 17 communicating segment internal carotid artery, 39 middle cerebral artery, two posterior cerebral artery, 42 posterior communicating artery, three posterior inferior cerebellar artery, and four superior cerebellar artery. One hundred and ninety-nine cases were balloon-assisted coiling with only case it not being utilized.
[
Retreatment postcoiling
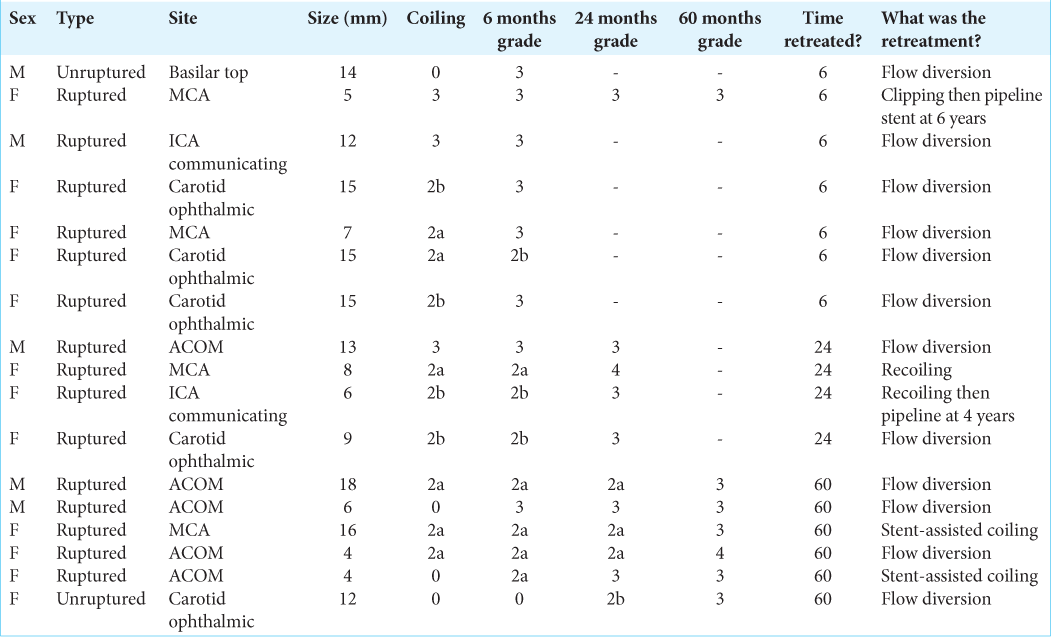
Overall 17/200 (8.5%) cases required retreatment within 5 years of initial coiling. 4/130 (3.1%) cases required retreatment within 5 years with initial Grade 0 at coiling, 6/24 cases (25%) of those Grade 2a, 4/20 cases (20%) Grade 2b, and 3/8 (38%) Grade 3. No cases of Grade 1 at coiling required retreatment. If aneurysm neck recurrence grading at 6 months follow up is used as a predictor for retreatment; 1% of grade 0 (1/100 cases), 0% of grade 1, 13% of grade 2a (5/38 cases), 23% of grade 2b (3/13 cases) and 89% of grade 3 cases (8/9) went onto having re-treatment within the follow up period of our study. 7/17 cases were retreated between after MDT discussion of the first follow-up MRA at 6 months. 4/17 cases were retreated after the second follow-up at 24 months and 6/17 after 60 months follow-up. Both aneurysm neck residues at the time of coiling at 6 months were statistically significant (P < 0.05) for risk of progression and retreatment, with greater correlation being seen with classification at 6 months follow-up. However, the degree of correlation was also similarly significant with the Raymond-Roy classification and no difference in predictability was seen between this newer and the Raymond-Roy classification. No statistical difference was seen between Grades 2a and 2b for predicting progression or risk of retreatment.
15/17 (88%) were initially ruptured aneurysms and 2/17 (12%) unruptured. However, this was not statistically significant (P = 0.30226). Of those retreated, 9/17 were large (≥11 mm), 5/17 medium (6–10 mm), and 3/17 small (≤5 mm) size; this was statistically significant (P < 0.05). 5/17 were carotid ophthalmic (29%), 5/17 ACOM (29%), 4/17 MCA (24%), 2/17 ICA communicating segment (12%), and 1/7 basilar top (6%). When accounting for total cases, the overall location for cases retreated was 5/9 (55.6%) were carotid ophthalmic, 5/55 ACOM (9.1%), 4/39 MCA (10.3%), 2/17 ICA communicating segment (11.8%), and 1/13 basilar top (7.7%); P < 0.05. 5/17 (29%) were male and 12/17 females (71%); gender was not a statistically significant factor for recurrence (P = 0.901). The location was statistically significant. [
DISCUSSION
Several factors have been previously been described in the literature as predictors of aneurysm recurrence, including incomplete aneurysm occlusion at coiling, packing density, large aneurysm size, and ruptured aneurysm prior to treatment.[
In our study, we found no significant increased risk of recurrence between Grades 2a and 2b at time of coiling and subsequent follow-up. There was low risk of recurrence and retreatment for Grade 0 or 1 at the time of coiling and at 6 months follow-up, whereas, as expected, high risk for Grade 3. Acute ruptured aneurysm cases accounted for almost 90% of those needing retreatment. Overall, 9.7% of ruptured aneurysms required retreatment versus 4.4% for unruptured. However, our sample size included a larger proportion of ruptured aneurysms and the differences accounted were not statistically significant. Aneurysms >10 mm accounted for 53% of overall cases that required retreatment. About 39.1% of large aneurysms (≥11 mm) needed retreatment, compared to 6.5% of medium sized and 3% of small aneurysms. Frequently, we would allow a small neck residue for ruptured or large aneurysms, particularly those with a wider neck with a view to later treat by flow diversion as part of a two-stage procedure. This allows safe occlusion of the aneurysm with the prevention of risk of rebleed. Striving for a perfect angiographic result may not be in the best interest of the patient with a ruptured aneurysm, leading to risks of possible resultant parent artery or side branch occlusion, coiling migration, infarction, and possibly death. In addition, treating with flow diversion carries a bleeding risk due to preloading on dual antiplatelets to avoid stent occlusion, which we feel outweighs the benefits in ruptured aneurysms due to the significant increased bleeding risk. Aneurysm location was also an important factor with 55.8% of carotid ophthalmic aneurysms being retreated with flow diversion. The PUFS trial demonstrated a 93.4% cure rate in patients retreated with pipeline device.[
Although aneurysm neck recanalization is an important factor in determining whether retreatment is offered, it should not be the only factor.[
For routine endovascular coiling at our institute, we routinely use HyperForm and HyperGlide balloons, and in our study, only case did not utilize a balloon. They provide a safety net in case of intraoperative aneurysm or vessel rupture, with inflation of the device providing a lifeline through temporary occlusion of the supplying vessel, thus helping to reduce the volume of blood loss before definitive treatment.[
Limitations of the study
The retrospective single center nature of the study and variation in treatment approaches among colleagues in different centers could limit the usefulness of a classification system. Direct comparison of residual aneurysm between angiogram DSA and MRA can lead to interpretation discrepancies despite each case being reviewed individually by two authors. Most interpretation discrepancies were between Grades 2a and 2b. A prospective study with longer follow-up of a greater proportion of participants could be useful for further research.
CONCLUSION
In our study, we found no significant difference in progressive recurrence or need for retreatment between Grades 2a and 2b. There was no statistical difference in the predictive value between the Raymond-Roy and modified classification, which limits its clinical application benefit. The Raymond-Roy classification with three basic grades appears to be sufficient in aiding recurrence management, with Grade 3 being high risk of rebleeding and requiring retreatment and Grades 1–2 being lower risk. However, we do feel that the addition of Grade 4 new aneurysms growth is useful as this is a different entity and likely would require treatment. Further larger multicenter studies investigating the role of subdivisions could be useful in the future to evaluate their clinical role.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2017. 127: 81-8
2. Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ, Murad MH, Wang Z. Risk factors for growth of intracranial aneurysms: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016. 37: 615-20
3. Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: Outcomes and incidence of late rebleeding. J Neurosurg. 1999. 90: 656-63
4. Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke. 2007. 38: 1538-44
5. Crobeddu E, Lanzino G, Kallmes DF, Cloft HJ. Review of 2 decades of aneurysm-recurrence literature, part 2: Managing recurrence after endovascular coiling. Am J Neuroradiol. 2013. 34: 481-5
6. Dorfer C, Gruber A, Standhardt H, Bavinzski G, Knosp K. Management of residual and recurrent aneurysms after initial endovascular treatment. Neurosurgery. 2012. 70: 537-54
7. Elijovich L, Higashida RT, Lawton MT, Duckwiler G, Giannotta S, Johnston SC. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: The CARAT study. Stroke. 2008. 39: 1501-6
8. Futchko J, Starr J, Lau D, Leach MR, Roark C, Pandey AS. Influence of smoking on aneurysm recurrence after endovascular treatment of cerebrovascular aneurysms. J Neurosurg. 2018. 128: 992-8
9. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: The Cerebral Aneurysm Rerupture after Treatment (CARAT) study. Stroke. 2008. 39: 120-5
10. Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001. 32: 485-91
11. Koopman I, Greving JP, van der Schaaf IC, van der Zwan A, Rinkel GJ, Vergouwen MD. Aneurysm characteristics and risk of rebleeding after subarachnoid haemorrhage. Eur Stroke J. 2019. 4: 153-9
12. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015. 385: 691-7
13. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009. 8: 427-33
14. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005. 366: 809-17
15. Naggara ON, White PM, Guilbert F, Roy D, Weill A, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: Systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010. 256: 887-97
16. Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A. Predictors and Impact of Aneurysm Rebleeding After Subarachnoid Hemorrhage. Arch Neurol. 2005. 62: 410-6
17. Ogilvy CS, Chua MH, Fusco MR, Reddy AS, Thomas AJ. Stratification of recanalization for patients with endovascular treatment of intracranial aneurysms. Neurosurgery. 2015. 76: 390-5
18. Okada T, Ishikawa T, Moroi J, Suzuki A. Timing of retreatment for patients with previously coiled or clipped intracranial aneurysms: Analysis of 156 patients with multiple treatments. Surg Neurol Int. 2016. 7: S40-8
19. Piotin M, Blanc R. Balloons and stents in the endovascular treatment of cerebral aneurysms: vascular anatomy remodeled. Front Neurol. 2014. 5: 41
20. Plowman RS, Clarke A, Clarke M, Byrne JV. Sixteen-year single-surgeon experience with coil embolization for ruptured intracranial aneurysms: Recurrence rates and incidence of late rebleeding. J Neurosurg. 2011. 114: 863-74
21. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003. 34: 1398-403
22. Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. 2007. 28: 1755-61
23. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001. 32: 1998-2004
24. Shapiro M, Babb J, Becske T, Nelson PK. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: A literature review. AJNR Am J Neuroradiol. 2008. 29: 1777-81
25. Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Balloon-assisted coil embolization of intracranial aneurysms: Incidence, complications, and angiography results. J Neurosurg. 2006. 105: 396-9
26. Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES. Guidelines for the management of patients with unruptured intracranial aneurysms: A guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2015. 46: 2368-400
27. Yu L, Yang X, Zhang Q, Zhang S, Zhang Y, Wang R. Management of recurrent intracranial aneurysms after coil embolization: A novel classification scheme based on angiography. J Neurosurg. 2019. 131: 1455-61