- Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Division of Stroke Prevention and Treatment, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Department of Cardiology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Department of Stroke and Cerebrovascular Diseases, University of Tsukuba Hospital, Tsukuba, Japan.
Correspondence Address:
Mikito Hayakawa, Division of Stroke Prevention and Treatment, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
DOI:10.25259/SNI_560_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryosuke Shintoku1, Mikito Hayakawa2, Tomoya Hoshi3, Sho Okune4, Takato Hiramine4, Toshihide Takahashi1, Hisayuki Hosoo1, Yoshiro Ito1, Aiki Marushima1, Eiichi Ishikawa1, Yuji Matsumaru1. Carotid artery stenosis concomitant with severe aortic stenosis treated by combination of staged angioplasty and transcatheter aortic valve implantation: A case report. 14-Oct-2022;13:469
How to cite this URL: Ryosuke Shintoku1, Mikito Hayakawa2, Tomoya Hoshi3, Sho Okune4, Takato Hiramine4, Toshihide Takahashi1, Hisayuki Hosoo1, Yoshiro Ito1, Aiki Marushima1, Eiichi Ishikawa1, Yuji Matsumaru1. Carotid artery stenosis concomitant with severe aortic stenosis treated by combination of staged angioplasty and transcatheter aortic valve implantation: A case report. 14-Oct-2022;13:469. Available from: https://surgicalneurologyint.com/surgicalint-articles/11930/
Abstract
Background: When severe aortic stenosis (AS) is concomitant with carotid stenosis, carotid artery stenting (CAS) will become a high-risk procedure because baroreceptor reflex-induced bradycardia and hypotension may cause irreversible circulatory collapse. When carotid stenosis-related misery perfusion is present, the risk of cerebral hyperperfusion syndrome increases after carotid revascularization. We report a case of severe carotid disease concomitant with severe AS successfully treated by a combination of staged angioplasty (SAP) and transcatheter aortic valve implantation (TAVI).
Case Description: An 86-year-old man presented with transient deterioration of mental status and sluggish responsiveness continuous from the previous day. Magnetic resonance imaging of the brain revealed a right putaminal infarction, occlusion of the right internal carotid artery (ICA), and severe stenosis of the left ICA. Severe AS was diagnosed and single-photon emission computed tomography showed misery perfusion at the bilateral ICA territories. We performed a staged treatment consisting of SAP for the left carotid stenosis and TAVI. A first-stage carotid angioplasty was performed, followed by TAVI 2 weeks later and second-stage CAS 1 week after that. There were no apparent periprocedural complications throughout the clinical course.
Conclusion: Combining SAP and TAVI may be an effective treatment option for severe carotid stenosis with misery perfusion concomitant with severe AS.
Keywords: Carotid artery stenting, Cerebral hyperperfusion syndrome, Severe aortic stenosis, Staged angioplasty, Transcatheter aortic valve implantation
INTRODUCTION
There is currently no consensus on the treatment strategy for severe carotid artery stenosis concomitant with severe aortic stenosis (AS). Carotid artery stenting (CAS) may worsen the systemic circulatory dynamics of patients with severe AS, sometimes leading to circulatory collapse due to bradycardia and hypotension induced by baroreceptor reflex during the revascularization procedure.[
CASE DESCRIPTION
An 86-year-old man with a history of hypertension, Type 2 diabetes mellitus, dyslipidemia, and permanent atrial fibrillation visited our hospital and presented with transient deterioration of mental status and mildly sluggish responsiveness, continuous since the previous day. At examination time, his consciousness level was almost clear, without obviously abnormal neurological findings. Diffusion-weighted magnetic resonance imaging of the brain revealed a new-onset cerebral infarction of the right putamen [
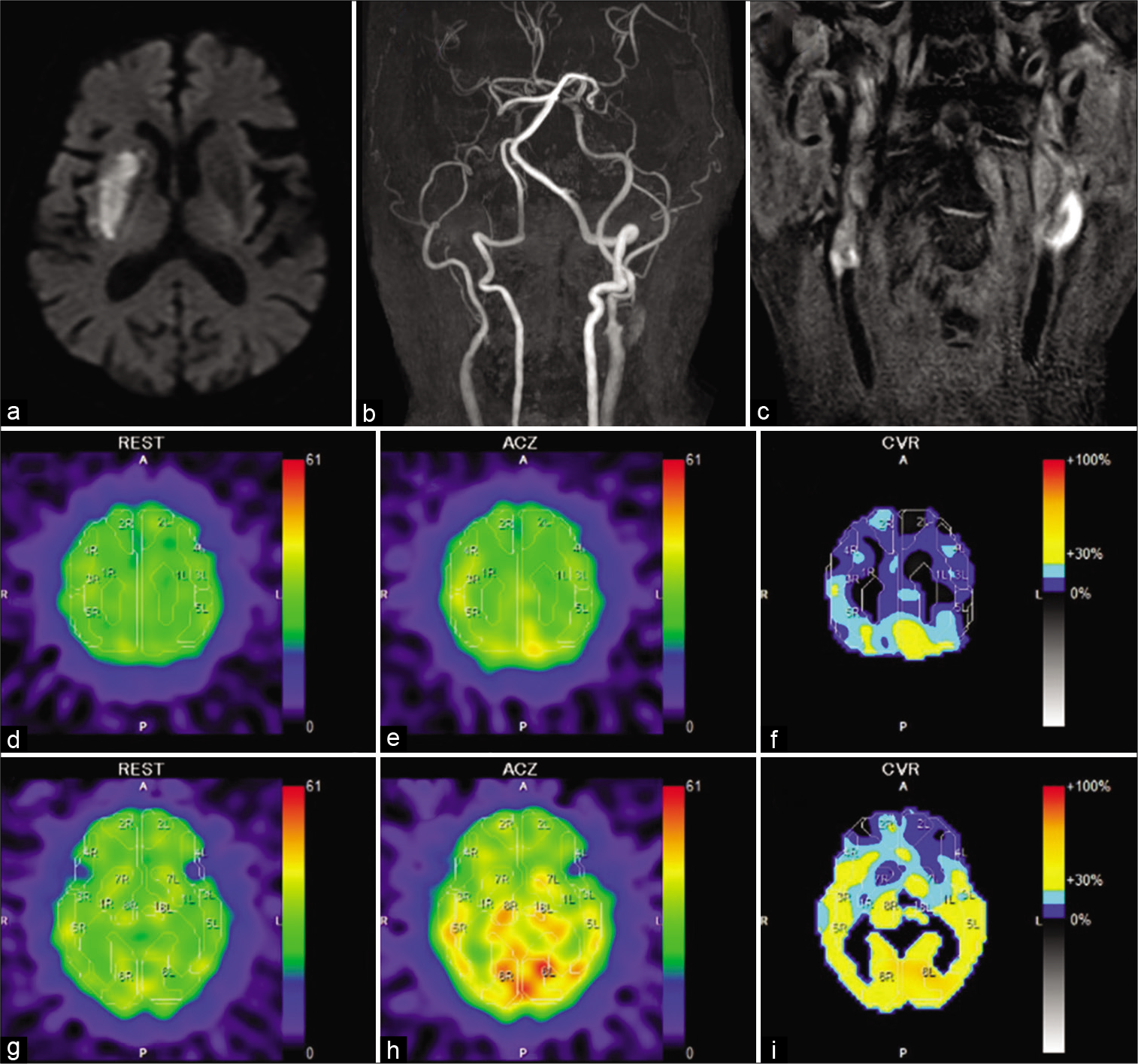
Figure 1:
(a) Diffusion-weighted magnetic resonance (MR) imaging of the brain at admission showing a newly onset cerebral infarction in the right putamen. (b) MR angiography showing occlusion of the right internal carotid artery (ICA) and severe stenosis of the left ICA at its cervical portion. (c) T1- weighted black blood MR imaging at the neck showing bright signal at the cervical portion of the left ICA. The plaque/muscle ratio was 3.39. (d-i) Preprocedural single-photon emission computed tomography (SPECT) using N-isopropyl-p-(123I) iodoamphetamine (123I-IMP) at resting state (d and g), after an acetazolamide challenge (e and h), and illustrations of cerebrovascular reactivity (CVR) using a color scale (f and i). The upper row indicates the centrum semiovale level and the lower row indicates the basal ganglia level. SPECT at resting state shows a decrease in cerebral blood flow (CBF) in the bilateral cerebral hemispheres (22–29 mL/100 g/min). SPECT after an acetazolamide challenge showing an insufficient increase of CBF, illustrations of CVR showing its extensive decline (7–26%), especially in the territories of the anterior cerebral artery and the superior branch of the middle cerebral artery in the bilateral cerebral hemispheres.
In this case, patient status under medical treatment was considered poor because of misery perfusion in the bilateral cerebral hemispheres[
Procedures
First-stage angioplasty
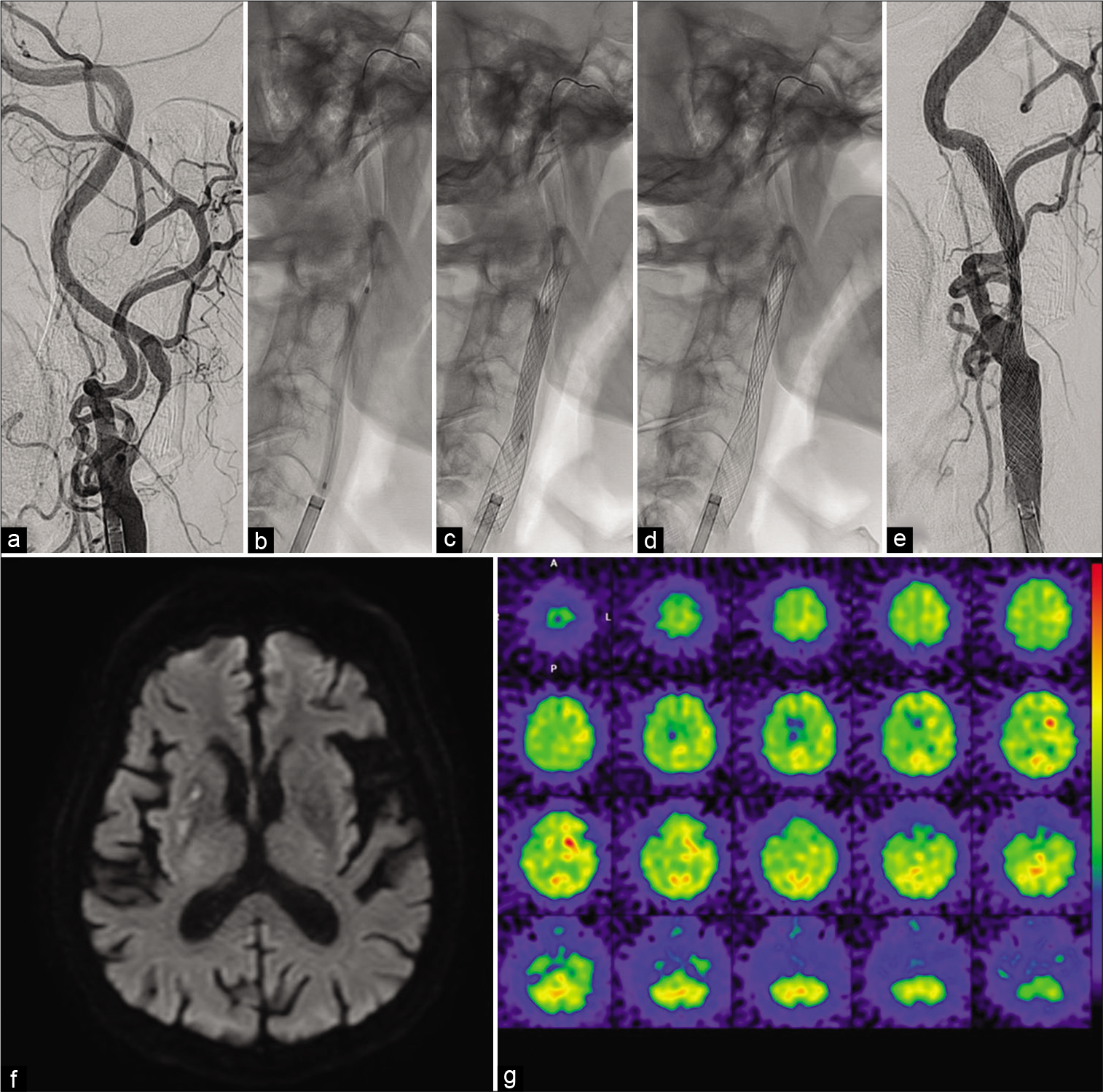
Under the administration of DAPT with apixaban, we performed first-stage angioplasty under local anesthesia on the 75th day after stroke onset. Because of a so-called bovine arch, we advanced a 6Fr. FUBUKI Dilator Kit (ASAHI. INTEC, Aichi, Japan) into the left common carotid artery (CCA) through a right radial artery approach [
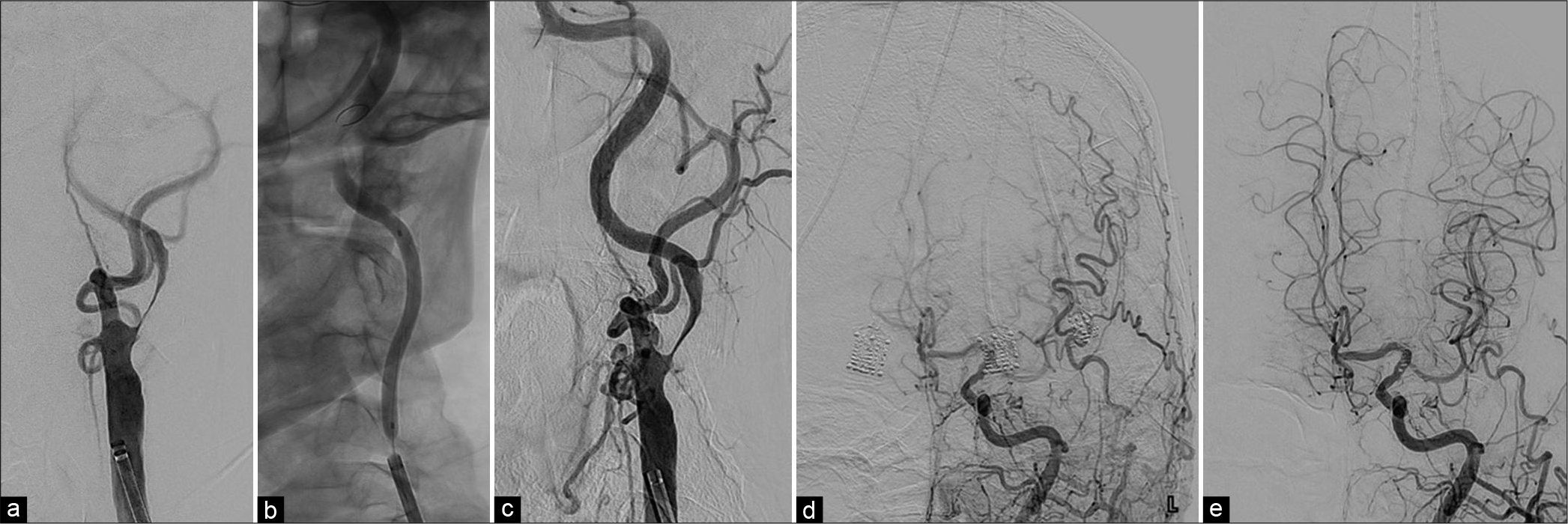
Figure 2:
(a and c) Left common carotid artery angiograms at the neck level (left anterior oblique view) before (a) and after (c) the first-stage angioplasty (b): Intraprocedural fluoroscopy of the first-stage angioplasty. Severe stenosis of the left ICA at its cervical portion with delayed opacification of the portion just distal to the stenosis being improved after angioplasty. (d and e) Left common carotid artery angiograms at the intracranial level (anteroposterior views) before (d) and after (e) the first-stage angioplasty. Opacification of territories fed by the left ICA including the right anterior cerebral artery becoming faster and clearer.
TAVI
Fourteen days after the first-stage angioplasty, TAVI was performed through the right femoral artery approach under general anesthesia [
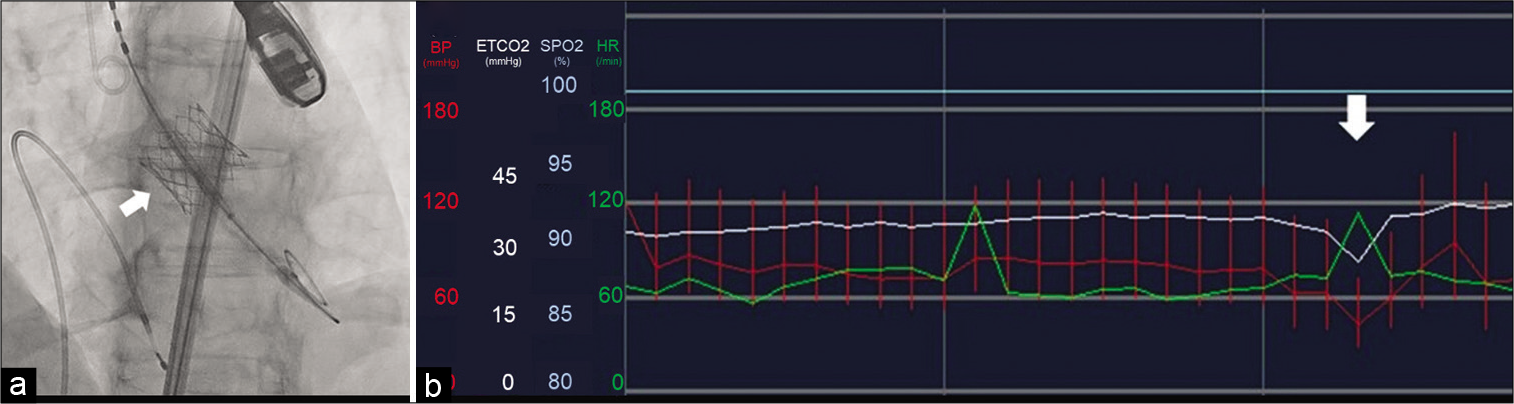
Figure 4:
(a) Intraprocedural fluoroscopy during transcatheter aortic valve implantation (TAVI) demonstrating the deployment of the valve device (white arrow). (b) Vital signs chart during general anesthesia for the TAVI procedure demonstrating a pronounced decrease of blood pressure during the steep increment of heart rate due to the rapid right ventricular pacing for deployment of the valve device (white arrow). The red bars and green lines represent the blood pressure and heart rate, respectively.
Second-stage CAS
Seven days after TAVI, we performed the second-stage CAS under local anesthesia, having resumed clopidogrel and apixaban from the day after TAVI. We advanced a 6Fr. FUBUKI Dilator Kit into the left CCA through a right brachial artery approach. The left carotid angiogram showed no apparent restenosis in the formerly angioplastied portion [
Figure 5:
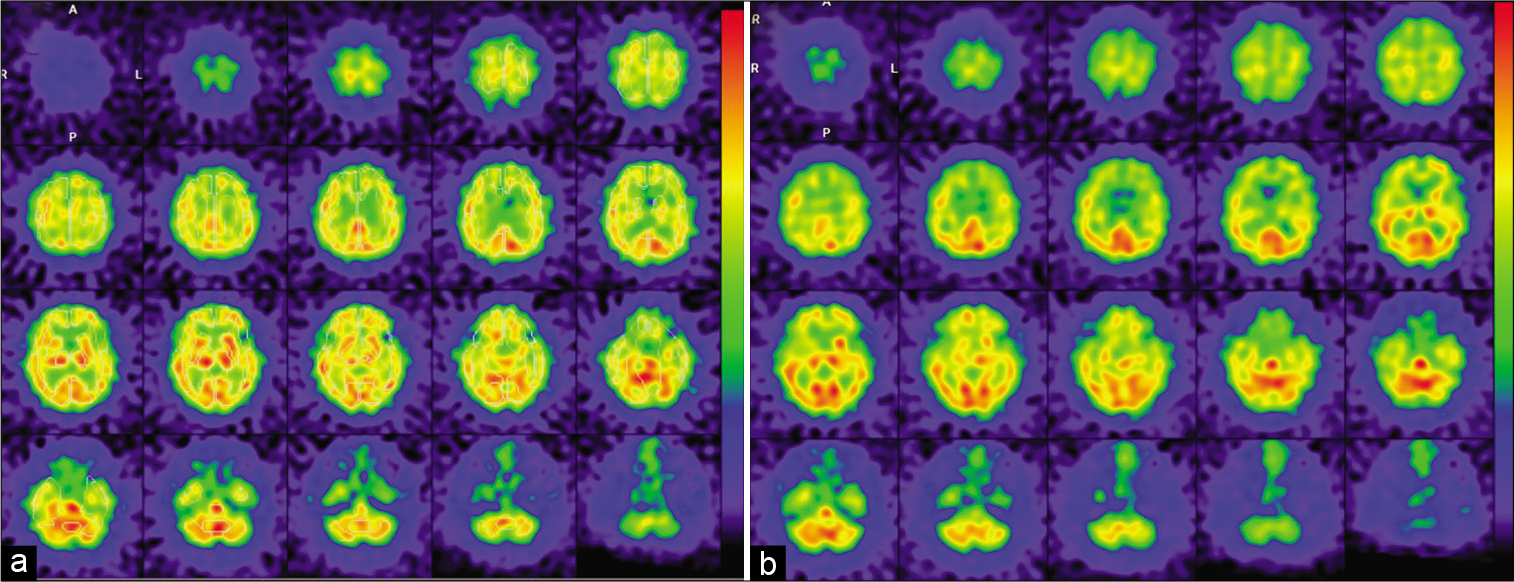
(a and e) Left common carotid artery angiograms at the neck level (left anterior oblique view) before (a) after (e) the second-stage carotid artery stenting (CAS). (b-d) Intraprocedural fluoroscopies during predilatation (b), postdilatation (c), and immediately after CAS procedure (d and f). Diffusion-weighted MR imaging of the brain on the day after the second-stage CAS showing no newly onset, abnormal, and hyperintense lesion. (g) SPECT on the day after the second-stage CAS showing a mild increase (recovery) of CBF in the territories of the left anterior and middle cerebral arteries that did not meet the criteria for hyperperfusion phenomenon.
DISCUSSION
We successfully treated a case of severe carotid disease with misery perfusion concomitant with severe AS by combining SAP and TAVI. To the best of our knowledge, this is the first case report of such a treatment strategy.
Optimal treatment strategies for patients with severe carotid stenosis combined with severe AS are still unclear. In general, CAS during severe AS should be avoided because of the risk of circulatory collapse and cardiac arrest due to bradycardia and hypotension.[
The key point in this case was the effectiveness of SAP in two ways: preserving CBF during TAVI and preventing CHS after CAS. SAP has been reported as an effective method against CHS in patients at high risk for CHS without increasing ischemic complications.[
Because improvement of misery perfusion was judged necessary for a safe TAVI, we prioritized first-stage angioplasty for the left carotid stenosis in our combined treatment strategy. Post-CAS bradycardia and hypotension result from carotid baroreceptor stimulation caused by balloon dilatation[
The target MLD in the first-stage angioplasty was considered to be 2.0 mm to avoid both CHS and ischemic complications.[
To obtain and maintain the optimal MLD, additional stenting using a detachable, rolled-sheet stent (Solitaire; Covidien, Irvine, CA, USA), or a balloon-expandable coronary stent at the first stage were reported.[
In the present case, TAVI was performed 2 weeks after the first-stage angioplasty and second-stage CAS was performed 1 week later. Uchida et al. showed that severely reduced CVR recovered to nearly normal values after 2 weeks.[
CONCLUSION
The combination of SAP and TAVI may be an effective treatment option for severe carotid stenosis with misery perfusion concomitant with severe AS.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Alexander Zaboronok and Dr. Bryan J. Mathis for professional and language revision.
References
1. Dangas G, Laird JR, Satler LF, Mehran R, Mintz GS, Larrain G. Postprocedural hypotension after carotid artery stent placement: Predictors and short-and long-term clinical outcomes. Radiology. 2000. 215: 677-83
2. Ezura M, Kimura N, Sakata H, Ishida T, Inoue T, Uenohara H. Carotid artery stenting using balloon-expandable coronary stent: Intentional use for staged angioplasty. J Neuroendovascular Ther. 2021. 15: 621-8
3. Gennari M, Trabattoni P, Bartorelli A, Agrifoglio M. A short report on single stage transcatheter aortic valve replacement and carotid stenting. Thorac Cardiovasc Surg Rep. 2017. 6: e37-9
4. Hayakawa M, Sugiu K, Yoshimura S, Hishikawa T, Yamagami H, Fukuda-Doi M. Effectiveness of staged angioplasty for avoidance of cerebral hyperperfusion syndrome after carotid revascularization. J Neurosurg. 2020. 132: 51-61
5. Hynes BG, Rodés-Cabau J. Transcatheter aortic valve implantation and cerebrovascular events: The current state of the art. Ann N Y Acad Sci. 2012. 1254: 151-63
6. Im SH, Han MH, Kim SH, Kwon BJ. Transcutaneous temporary cardiac pacing in carotid stenting: Noninvasive prevention of angioplasty-induced bradycardia and hypotension. J Endovasc Ther. 2008. 15: 110-6
7. Kaku Y, Yoshimura SI, Kokuzawa J. Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. Am J Neuroradiol. 2004. 25: 1403-8
8. Kar S, Krishnaswamy A, Shishehbor MH, Cam A, Tuzcu EM, Bhatt DL. Safety and efficacy of carotid stenting in individuals with concomitant severe carotid and aortic stenosis. EuroIntervention. 2010. 6: 492-7
9. Kleiman NS, Maini BJ, Reardon MJ, Conte J, Katz S, Rajagopal V. Neurological events following transcatheter aortic valve replacement and their predictors: A report from the corevalve trials. Circ Cardiovasc Interv. 2016. 9: e003551
10. Kochar A, Li Z, Harrison JK, Hughes GC, Thourani VH, Mack MJ. Stroke and cardiovascular outcomes in patients with carotid disease undergoing transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2018. 11: e006322
11. Mendelsohn FO, Weissman NJ, Lederman RJ, Crowley JJ, Gray JL, Phillips HR. Acute hemodynamic changes during carotid artery stenting. Am J Cardiol. 1998. 82: 1077-81
12. Rutgers DR, Klijn CJ, Kappelle LJ, Eikelboom BC, van Huffelen AC, van der Grond J. Sustained bilateral hemodynamic benefit of contralateral carotid endarterectomy in patients with symptomatic internal carotid artery occlusion. Stroke. 2001. 32: 728-34
13. Thirumala PD, Muluk S, Udesh R, Mehta A, Schindler J, Mulukutla S. Carotid artery disease and periprocedural stroke risk after transcatheter aortic valve implantation. Ann Card Anaesth. 2017. 20: 145-51
14. Uchida K, Yoshimura S, Shirakawa M, Shindo S, Egashira Y, Iwama T. Experience of staged angioplasty to avoid hyperperfusion syndrome for carotid artery stenosis. Neurol Med Chir (Tokyo). 2015. 55: 824-9
15. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2018. 53: 34-78
16. Yamauchi H, Higashi T, Kagawa S, Nishii R, Kudo T, Sugimoto K. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease?. Brain. 2012. 135: 2515-26
17. Yoshimura S, Kitajima H, Enomoto Y, Kiyofumi Y, Iwama T. Staged angioplasty for carotid artery stenosis to prevent postoperative hyperperfusion. Neurosurgery. 2009. 64: 122-9
18. You C, Zhang X, Wu Y, Sun W, Li J, Zhang L. Solitaire stents deployment may reduce ischemic events in staged angioplasty for severe carotid stenosis. Vascular. 2021. 29: 535-42